107 Joint Disorders
• A white blood cell count higher than 50,000/mm3 is suggestive of a septic joint; however, lower counts do not rule out this diagnosis. If the index of clinical suspicion for a septic joint is high but the test results are nondiagnostic, the disorder should be treated as an infection.
• Severely painful acute monarticular arthritis, with or without fever, is highly likely to be a septic joint; it is an emergency requiring parenteral antibiotics and possible surgical intervention.
• Joint prostheses, diabetes, rheumatoid arthritis, concurrent infection, and age older than 80 years are significant risk factors for bacterial joint infections. Acute problems in a prosthetic joint merit immediate discussion with an orthopedic surgeon.
• A sexually active young adult with an inflamed, painful joint is considered to have gonococcal arthritis until proved otherwise and should be treated accordingly.
• Gout and pseudogout are crystal-induced arthritides that although benign, may cause significant pain and morbidity. The patient’s pain can be significantly improved by anesthetic and steroid injection after arthrocentesis.
• Overlying soft tissue infection may preclude arthrocentesis or warrant a different approach.
• Syncope may be a warning of high risk for sudden cardiac death in patients with systemic inflammatory arthritides.
• Cervical spine disease is common with rheumatoid arthritis and osteoarthritis and must be considered before any attempt at endotracheal intubation that involves forced flexion.
• Serious complications of arthritides are rare but may be life- or limb-threatening.
Arthritis
Epidemiology
An estimated 20 million people in the United States have osteoarthritis (OA), and 2 to 3 million have rheumatoid arthritis (RA). The total cost of medical care, lost work time, and disability for OA alone is estimated to run 2% of the gross national product. The most common cause of joint pain is OA, but the most crucial challenge is identification of a septic joint. Septic arthritis occurs in all age groups but is more common in children than in adults, and about 10% of patients with an acutely painful joint will be found to have infection.1
Pathophysiology
The structure of diarthrodial joints (the most common type) includes the synovium, synovial fluid, articular cartilage, intraarticular ligaments, joint capsule, and juxtaarticular subchondral bone. The delicate synovium provides oxygen and nutrients to cartilage and produces lubricants. Articular cartilage deforms under mechanical load to minimize stress and provides a smooth surface for joint motion with minimal friction. Causes of joint disorders (Box 107.1) often overlap. Cumulative microdamage and remodeling occur with use and aging. Mechanical or metabolic disturbances may lead to a secondary inflammatory response, or an inflamed structure (e.g., a tendon) may rupture. Arthrosis is due to a mechanical insult, whereas arthritis is due to inflammation of the synovium. With inflammation comes white blood cell (WBC) infiltration, release of cytokines (e.g., tumor necrosis factor-α [TNF-α], interleukins) and other inflammatory mediators, and proliferation of cells or tissue. Edema collects around the joint, which causes stiffness. With prolonged inflammation, erosion of bone and destruction of the joint eventually occur and can produce deformity and chronic disability.
Presenting Signs and Symptoms
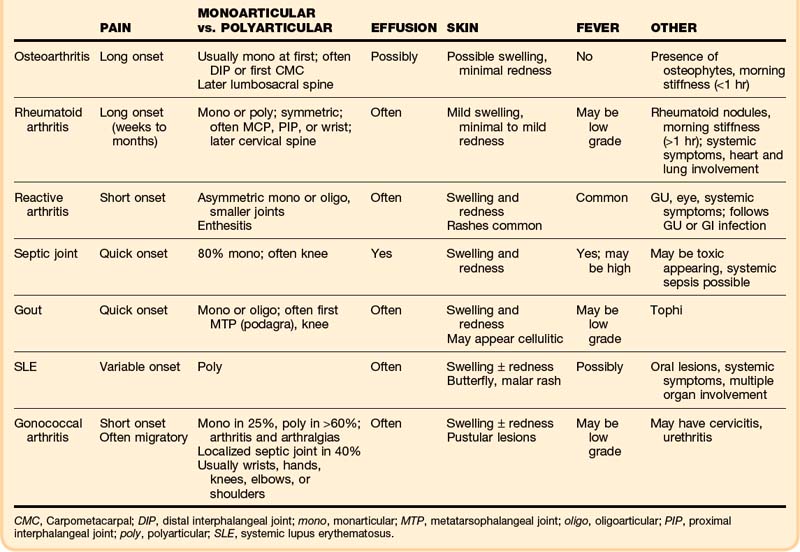
The clinical findings can narrow the potential cause of a patient’s symptoms (Table 107.1). Initial assessment must determine whether the anatomic site of the problem is the joint and then a general category of the disease, either inflammatory (septic versus aseptic) or noninflammatory (mechanical). A red, hot, swollen, painful joint is the classic finding with septic and other inflammatory arthritides. Arthritis patients may also have serious nonarticular complications of their disease or its treatment.
The onset and pattern of pain are important to determine (Box 107.2). Mechanical pain is worse with use, rapidly relieved with rest, and often least in the morning. If present, morning stiffness resolves quickly. Rapid onset over a period of minutes suggests trauma, internal derangement, or a loose fragment in the joint. Inflammatory pain is often worse with use as well, but not so quickly relieved with rest, and is commonly associated with morning stiffness (short duration with OA, prolonged with RA). “Gelling” (stiffness and immobility) after sitting in one position occurs with either type. Widespread pain with stiffness is typically due to inflammatory arthritis or fibromyalgia. Subjective pain without joint findings on examination is termed arthralgia. If the patient has tried medications without relief, the dosage should be determined because inadequate dosing is common.
Box 107.2 Key Historical Points
What is the source and type of pain?
What is the OPQRST (onset, palliation/aggravation, quality, radiation, severity, timing)?
Medications, especially thiazides (can increase serum uric acid), isoniazid, procainamide, and hydralazine (can precipitate lupus)?
Current and previous treatments? Nontraditional remedies? Results?
The musculoskeletal examination attempts to identify the exact site of the problem—joint versus bone, muscle, periarticular, or superficial skin pain. Particular joint involvement may aid in making the diagnosis (Box 107.3). True joint pain is usually diffuse on palpation and increases with active and passive motion. Periarticular inflammation (tendonitis, bursitis, cellulitis) is generally more focal, with pain reproduced only by certain movements—most often resisted active contraction or passive stretching of the involved muscles or tendons and usually only toward one side.
Box 107.3 Articular Diseases Associated with Joint Location
By Specific Joint or Joints
First metatarsophalangeal (MTP): Gout
Knee: Septic arthritis, pseudogout, gout, OA
Metacarpophalangeal, MTP, proximal interphalangeal, tarsometatarsal, and cervical spine: RA
Distal and proximal interphalangeal, first carpometacarpal, knee, hip, cervical and lumbosacral spine: OA
Sternoclavicular: Injection drug abusers with septic joints
Differential Diagnosis and Medical Decision Making
Mechanical, inflammatory, or metabolic causes of arthritis may be present, and the whole picture must be considered in narrowing the lengthy differential diagnosis (Box 107.4). A new diagnosis of a specific type of inflammatory arthritis may not be possible in one visit, but recognition that the case is inflammatory is important for interim care. The severity of a patient’s discomfort will determine the urgency of analgesia, which may be initiated well before refining the list of possible diagnoses.
Box 107.4 Differential Diagnoses for Acute Arthritis
Noninflammatory Joint Disorder
Avascular necrosis of the hip (including Legg-Calvé-Perthes disease*)
Charcot (neuropathic) arthropathy
Hemarthrosis, hemophilic arthropathy
Hypertrophic pulmonary osteoarthropathy
Inherited storage diseases (e.g., Gaucher)
Liquid lipid microsphere disease
Pigmented villonodular synovitis
Slipped capital femoral epiphysis
Inflammatory Joint Disorder
Connective tissue diseases: systemic lupus erythematosus, scleroderma, Sjögren syndrome, mixed connective tissue disease
Crystal deposition: gout, pseudogout
Drug reaction (serum sickness)
Juvenile idiopathic arthritis and subtypes*
Osteoarthritis, degenerative joint disease
Polymyalgia rheumatica with joint involvement
Seronegative spondyloarthropathies*
Diagnostic Studies
Blood tests are rarely diagnostic in patients with synovial disorders but may be ordered sparingly to assist in management decisions. A complete blood count (CBC) and basic chemistry profile will identify anemia, an elevated WBC count, and renal dysfunction (which affects selection of medications). The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are nonspecific but useful markers of inflammation. Coagulation studies are needed only if a patient is taking anticoagulants or a bleeding disorder is suspected. Creatine phosphokinase is helpful if muscle pain or weakness is detected. If clinical assessment raises concern for other autoimmune or systemic diseases, additional screening for multiorgan involvement with urinalysis, liver enzymes, electrocardiogram, and chest films may help. Serologic testing (e.g., rheumatoid factor [RF], antinuclear antibody, and Lyme serology, depending on the clinical impression) is generally done in follow-up settings.2
Plain radiographs are ordered if a fracture, foreign body, septic joint, or tumor is suspected. If the initial films show no fracture but suspicion remains, films repeated in 1 to 2 weeks may show callus formation or abnormal alignment. Radiographic findings may also assist in diagnosing the type of arthritis (Box 107.5),3 although they may remain normal early in the course. The presence of degenerative changes in a painful joint supports the clinical suspicion of OA as the cause, but such changes also become common with age, even in asymptomatic joints; conversely, a normal film does not rule out OA. Similarly, calcified fibrocartilage is often found in patients with calcium pyrophosphate deposition (CPPD) disease but is common in asymptomatic patients as well. Ultrasonography is useful in confirming joint effusion, especially in joints that are difficult to assess, such as the hip. Other modalities are rarely indicated in the emergency department (ED). Although magnetic resonance imaging (MRI) can distinguish synovitis from effusion and identify rotator cuff tears or be used to evaluate ligament trauma, emergency MRI or computed tomography (CT) is indicated only if a severe joint complication is strongly suspected or if axial skeletal pain merits evaluation for stenosis or metastatic disease.
Box 107.5
“Seconds” Mnemonic for Radiographic Evaluation of Arthritis
Soft tissue swelling—Nonspecific, often seen with acute arthritides such as gout, pseudogout, and septic arthritis, as well as with tuberculous arthritis; also present in trauma
Erosions—May be present in late rheumatoid arthritis as a result of the pannus eroding into articular cartilage and bone
Calcification—In late pseudogout, there may be linear calcification in cartilage
Osteoporosis—Sometimes present in late septic arthritis as a result of joint destruction (about 8 to 10 days of disease before changes are evident on plain films). Osteoporosis or periarticular bone may be seen with late rheumatoid arthritis but not with pseudogout or osteoarthritis
Narrowing of the joint space—Present in late septic arthritis; asymmetric narrowing is consistent with late pseudogout and osteoarthritis; symmetric narrowing is consistent with late rheumatoid arthritis. Joint space is typically preserved with tuberculous arthritis
Deformity—In late septic arthritis, subchondral bone destruction and periosteal new bone may be visualized; in late pseudogout and osteoarthritis, changes may include sclerosis, osteophyte formation, and subchondral cyst formation
Adapted from Beachley MC, Franklin JW, Ostlund W, et al. Radiology of arthritis. Prim Care 1993;20:771-94.
Arthrocentesis with synovial fluid analysis is an important diagnostic and therapeutic procedure for joint disease (see the Tips and Tricks box and Box 107.6). It is the only reliable means to rule out a septic joint, and it is essential in acute monarthritis to look for joint infection, crystals, or hemarthrosis. Possible complications of arthrocentesis include introduction of infection into the joint space, hemarthrosis, and adverse reactions to medications. Arthrocentesis of prosthetic joints is best done with orthopedic consultation.
Box 107.6 Arthrocentesis
Indications
Diagnosis of nontraumatic joint disease by synovial fluid analysis
Diagnosis of ligamentous or bony injury by confirmation of blood in the joint
Establishment of the existence of an intraarticular fracture by the presence of blood with fat globules in the joint
Relief of pain accompanying acute hemarthrosis or a tense effusion
Local instillation of medications
Obtaining fluid for analysis (culture, cell count, crystal studies)
Tips and Tricks
Joint Fluid Collection
Identify and mark landmarks before infiltration with an anesthetic.
Preprocedure use of an ice pack will decrease pain.
Support the joint in a position of comfort during and after the procedure.
Contact the laboratory technicians before collecting the fluid to verify the following:
Use sonographic localization of joint fluid.
Prepare the area thoroughly with the antiseptic of choice. Use sterile gloves and equipment.
Use an 18- to 22-gauge needle depending on the size of the joint; smaller needles may not be sufficient to collect joint fluid.
Attachment of extension tubing between the needle hub and the syringe helps decrease movement of the needle in the joint space and makes changing syringes with large-volume arthrocentesis and injection of medications into the joint space easier. Tubing must be flushed when injecting corticosteroids so that the full dose actually enters the joint space.
Collect enough fluid for appropriate testing (this is not an easily repeated procedure).
Send fluid for a cell count and differential, Gram stain and culture, crystals, glucose, and viscosity.
Seeding the fluid into blood culture flasks immediately after aspiration may increase the yield.
Have the patient rest the joint for 12 to 24 hours after the injection of corticosteroids.
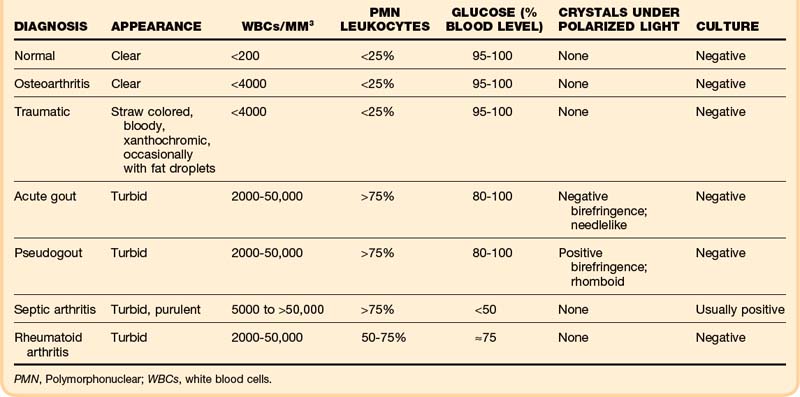
Normal synovial fluid is clear and yellow in appearance. In degenerative joint disease the fluid itself is normal and thus remains clear. Bloody fluid suggests hemarthrosis. Fat droplets may confirm a fracture. Turbid fluid is observed in inflammatory conditions: gout, pseudogout, and septic, rheumatoid and seronegative arthritides (Table 107.2).
Crystal analysis is performed under compensated polarizing microscopy. In patients with acute gout, monosodium urate crystals are present inside neutrophils in fluid from the affected joint. The crystals are typically needle shaped and appear yellow when parallel to the compensator; this is negative birefringence. Sensitivity is at least 85%, and specificity for gout is 100%.4 In pseudogout, the crystals are positively birefringent (blue when parallel to the compensator), usually rhomboid shaped, and also phagocytized by neutrophils. Acute gouty arthritis may occasionally coexist with septic arthritis or pseudogout.
Glucose may be decreased relative to serum glucose in severe inflammatory disorders: down to less than 50% of the serum glucose level in septic arthritis and 50% to 75% in rheumatoid and seronegative arthritides. However, evidence suggests that chemistry studies on joint fluid should be discouraged because their results may be misleading or redundant.5
Although a joint WBC count higher than 50,000/mm3 is generally said to be positive for infection, septic arthritis can occur with lower joint WBC counts, especially early in infection (36% of patients with septic arthritis had joint WBC counts lower than 50,000/mm3).6 In addition, patients with inflammatory arthritides such as RA, gout, and pseudogout may have very high joint WBC counts. Thus fluid must also be sent for Gram stain and culture. The yield is increased by immediate plating in the laboratory and perhaps by inoculating blood culture bottles with joint fluid in the ED. The serum WBC count, ESR, and joint WBC count are extremely variable in adults with septic arthritis.7 In the absence of a positive Gram stain, the ED clinician must consider the whole picture when determining the probability of septic arthritis.
Treatment
Removal of fluid from a joint effusion provides considerable relief. Intraarticular corticosteroids (e.g., triamcinolone hexacetonide, ranging from 5 mg in a finger joint to 40 mg in a large joint, or methylprednisolone, 2 to 5 mg in small joints and 10 to 25 mg in large joints) are recommended for effusions unless infection is suspected. The patient should be informed that the pain relief with corticosteroids typically begins in 1 to 2 days, peaks at about 1 week, and lasts for 1 week to a few months, during which time compliance with adjunctive measures helps prevent recurrence. Although minimal evidence supports the concern, repeated steroid use traditionally raises concern over cartilage damage, so use in the same joint is limited to every 3 to 4 months. Long-acting local anesthetic may be added to the injection for same-day short-term relief.8,9
Follow-up, Next Steps in Care, and Patient Education
Serious situations may warrant admission (Box 107.7). Patients with joint infections require admission and early consultation with an orthopedic surgeon or rheumatologist.
Box 107.7 Emergency Department Disposition Decisions
When to Admit
Inability to control pain without parenteral analgesics
Acute inability to ambulate despite treatment in the emergency department (ED)
Inability to care for self at home (physical and occupational therapy; social, placement issues)
Need for urgent operative intervention
Need for parenteral medications
Possible infection in a prosthetic joint
High-risk complications (e.g., acute renal failure, systemic vasculitis, cardiac involvement, hypoxic lung disease)
Indications for Rheumatology or Orthopedic Outpatient Follow-up
Time Line for Follow-up
In 2 to 3 days: Significant inflammation, serious risk if diagnostic delay, culture results to be checked (if timely outpatient visit not possible, schedule return to the ED)
Within 1 week: Lupus flare (no central nervous system or renal involvement; laboratory results not seriously altered); consultation with a rheumatologist for a possible increase in steroids
In 1 to 2 weeks: Patient with rheumatologic disease sick enough to merit an ED visit but not severely ill; intraarticular corticosteroids administered in the ED; most inflammatory arthritides (not septic)
In 2 to 4 weeks: Chronic, mild, or noninflammatory complaints
Topical analgesics may help, especially if only a single joint is problematic. Topical NSAIDs (diclofenac or ketoprofen, but not salicylates) and capsaicin (thin film of 0.025% cream applied four times per day) have been shown in trials to be beneficial, but maximal relief may take 3 to 4 weeks. Topical NSAIDs avoid the GI and renal complications of oral agents.10,11 Lidocaine patches are another option, although unstudied.
Appropriate lifestyle recommendations for patients with chronic arthritis are to stay as active as possible with daily activities and reasonable exercise programs. Physical and occupational therapy12 may contribute greatly to improved quality of life and ability to maintain independence in self-care but are usually arranged by the continuity physician. Initial range-of-motion (ROM) and later strengthening and aerobic exercise regimens are recommended; swimming pool exercise programs are quite helpful. Obese patients with lower extremity arthritis should be educated about the importance of weight reduction.
![]() Patient Teaching Tips
Patient Teaching Tips
At Discharge
Instruct patients to return to the emergency department (ED) for temperatures higher than 101° F or if the redness or swelling spreads; also state specific problems to watch out for—for example, patients given nonsteroidal antiinflammatory drugs should “stop the medicine and come to the ED if you have black or bloody stools.”
Educate patients about their specific condition. Examples of teaching points include the following:
Understanding plus compliance with the recommended medication dose and directions is important for a good outcome. Patients with inflammatory arthritis may benefit from continuing the antiinflammatory medications even after the pain improves.
Follow-up visits are important for a good outcome:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree