Key Concepts
 Repetitive administration of barbiturates (eg, infusion of thiopental for “barbiturate coma” and brain protection) saturates the peripheral compartments, minimizing any effect of redistribution, and rendering the duration of action more dependent on elimination. This is an example of context sensitivity.
Repetitive administration of barbiturates (eg, infusion of thiopental for “barbiturate coma” and brain protection) saturates the peripheral compartments, minimizing any effect of redistribution, and rendering the duration of action more dependent on elimination. This is an example of context sensitivity.
 Barbiturates constrict the cerebral vasculature, causing a decrease in cerebral blood flow, cerebral blood volume, and intracranial pressure.
Barbiturates constrict the cerebral vasculature, causing a decrease in cerebral blood flow, cerebral blood volume, and intracranial pressure.
 Although apnea may be relatively uncommon after benzodiazepine induction, even small intravenous doses of diazepam and midazolam have resulted in respiratory arrest.
Although apnea may be relatively uncommon after benzodiazepine induction, even small intravenous doses of diazepam and midazolam have resulted in respiratory arrest.
 In contrast to other anesthetic agents, ketamine increases arterial blood pressure, heart rate, and cardiac output, particularly after rapid bolus injections.
In contrast to other anesthetic agents, ketamine increases arterial blood pressure, heart rate, and cardiac output, particularly after rapid bolus injections.
 Induction doses of etomidate transiently inhibit enzymes involved in cortisol and aldosterone synthesis. Etomidate was often used in the past for ICU sedation before reports of its consistent ability to produce adrenocortical suppression in that circumstance appeared.
Induction doses of etomidate transiently inhibit enzymes involved in cortisol and aldosterone synthesis. Etomidate was often used in the past for ICU sedation before reports of its consistent ability to produce adrenocortical suppression in that circumstance appeared.
 Propofol formulations can support the growth of bacteria, so sterile technique must be observed in preparation and handling. Propofol should be administered within 6 h of opening the ampule.
Propofol formulations can support the growth of bacteria, so sterile technique must be observed in preparation and handling. Propofol should be administered within 6 h of opening the ampule.
Intravenous Anesthetics: Introduction
General anesthesia began with inhaled agents but now can be induced and maintained with drugs that enter the patient through a wide range of routes. Drug administration can be oral, rectal, transdermal, transmucosal, intramuscular, or intravenous for the purpose of producing or enhancing an anesthetic state. Preoperative sedation of adults is usually accomplished by way of oral or intravenous routes. Induction of general anesthesia in adults usually includes intravenous drug administration. Effective topical anesthesia with EMLA (eutectic mixture of local anesthetic) cream, LMX (plain lidocaine cream 4% and 5%), or 2% lidocaine jelly has increased the ease of intravenous inductions in children. Maintenance of general anesthesia is feasible with a total intravenous anesthesia (TIVA) technique. This chapter focuses on the intravenous agents used to produce hypnosis, including barbiturates, benzodiazepines, ketamine, etomidate, and propofol.
Barbiturates
Barbiturates depress the reticular activating system in the brainstem, which controls multiple vital functions, including consciousness. In clinical concentrations, barbiturates more potently affect the function of nerve synapses than axons. Their primary mechanism of action is believed to be through binding to the γ-aminobutyric acid type A (GABAA) receptor. Barbiturates potentiate the action of GABA in increasing the duration of openings of a chloride-specific ion channel.
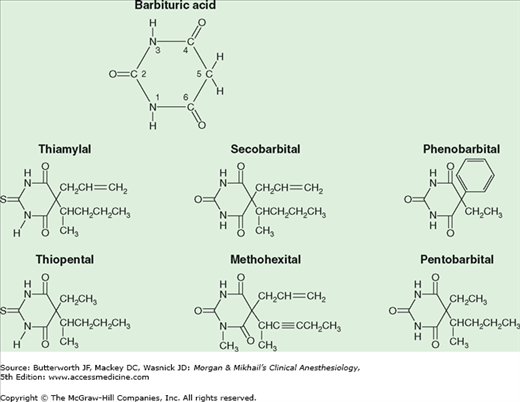
Barbiturates are derived from barbituric acid (Figure 9-1). Substitution at carbon C5 determines hypnotic potency and anticonvulsant activity. A long-branched chain conveys more potency than does a short straight chain. Likewise, the phenyl group in phenobarbital is anticonvulsive, whereas the methyl group in methohexital is not. Replacing the oxygen at C2 (oxybarbiturates) with a sulfur atom (thiobarbiturates) increases lipid solubility. As a result, thiopental and thiamylal have a greater potency, more rapid onset of action, and shorter durations of action (after a single “sleep dose”) than pentobarbital. The sodium salts of the barbiturates are water soluble but markedly alkaline (pH of 2.5% thiopental >10) and relatively unstable (2-week shelf-life for 2.5% thiopental solution). Concentrations greater than recommended cause an unacceptable incidence of pain on injection and venous thrombosis.
In clinical anesthesiology, thiopental, thiamylal, and methohexital were frequently administered intravenously for induction of general anesthesia in adults and children (prior to the introduction of propofol). Rectal thiopental or, more often, methohexital has been used for induction in children, and intramuscular (or oral) pentobarbital was often used in the past for premedication of all age groups.
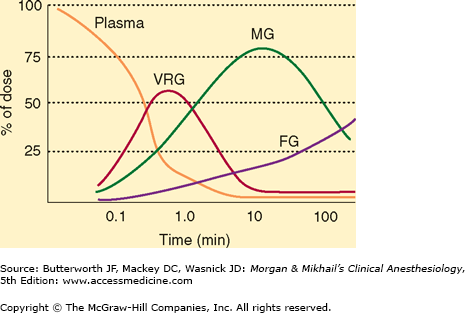
The duration of sleep doses of the highly lipid-soluble barbiturates (thiopental, thiamylal, and methohexital) is determined by redistribution, not by metabolism or elimination. For example, although thiopental is highly protein bound (80%), its great lipid solubility and high nonionized fraction (60%) account for rapid brain uptake (within 30 s). If the central compartment is contracted (eg, hypovolemic shock), if the serum albumin is low (eg, severe liver disease or malnutrition), or if the nonionized fraction is increased (eg, acidosis), larger brain and heart concentrations will be achieved for a given dose. Redistribution to the peripheral compartment—specifically, the muscle group—lowers plasma and brain concentration to 10% of peak levels within 20-30 min (Figure 9-2). This pharmacokinetic profile correlates with clinical experience—patients typically lose consciousness within 30 s and awaken within 20 min.
Figure 9-2
Distribution of thiopental from plasma to the vessel-rich group (VRG; brain, heart, liver, kidney, endocrine glands), to the muscle group (MG), and finally to the fat group (FG). (Modified and reproduced, with permission, from Price HL et al: The uptake of thiopental by body tissues and its relation to the duration of narcosis. Clin Pharmacol Ther 1960;1:16.)
The minimal induction dose of thiopental will depend on body weight and age. Reduced induction doses are required for elderly patients primarily due to slower redistribution. In contrast to the rapid initial distribution half-life of a few minutes, elimination of thiopental is prolonged (elimination half-life ranges of 10-12 h). Thiamylal and methohexital have similar distribution patterns, whereas less lipid-soluble barbiturates have much longer distribution half-lives and durations of action after a sleep dose.  Repetitive administration of barbiturates (eg, infusion of thiopental for “barbiturate coma” and brain protection) saturates the peripheral compartments, minimizing any effect of redistribution, and rendering the duration of action more dependent on elimination. This is an example of context sensitivity.
Repetitive administration of barbiturates (eg, infusion of thiopental for “barbiturate coma” and brain protection) saturates the peripheral compartments, minimizing any effect of redistribution, and rendering the duration of action more dependent on elimination. This is an example of context sensitivity.
Barbiturates are principally biotransformed via hepatic oxidation to inactive water-soluble metabolites. Because of greater hepatic extraction, methohexital is cleared by the liver more rapidly than thiopental. Although redistribution is responsible for the awakening from a single sleep dose of any of these lipid-soluble barbiturates, full recovery of psychomotor function is more rapid following methohexital due to its enhanced metabolism.
Increased protein binding decreases barbiturate glomerular filtration, whereas increased lipid solubility tends to increase renal tubular reabsorption. Except for the less protein-bound and less lipid-soluble agents such as phenobarbital, renal excretion is limited to water-soluble end products of hepatic biotransformation. Methohexital is excreted in the feces.
Intravenous bolus induction doses of barbiturates cause a decrease in blood pressure and an increase in heart rate. Hemodynamic responses to barbiturates are reduced by slower rates of induction. Depression of the medullary vasomotor center produces vasodilation of peripheral capacitance vessels, which increases peripheral pooling of blood, mimicking a reduced blood volume. Tachycardia following administration is probably due to a central vagolytic effect and reflex responses to decreases in blood pressure. Cardiac output is often maintained by an increased heart rate and increased myocardial contractility from compensatory baroreceptor reflexes. Sympathetically induced vasoconstriction of resistance vessels (particularly with intubation under light planes of general anesthesia) may actually increase peripheral vascular resistance. However, in situations where the baroreceptor response will be blunted or absent (eg, hypovolemia, congestive heart failure, β-adrenergic blockade), cardiac output and arterial blood pressure may fall dramatically due to uncompensated peripheral pooling of blood and direct myocardial depression. Patients with poorly controlled hypertension are particularly prone to wide swings in blood pressure during anesthesia induction. The cardiovascular effects of barbiturates therefore vary markedly, depending on rate of administration, dose, volume status, baseline autonomic tone, and preexisting cardiovascular disease. A slow rate of injection and adequate preoperative hydration attenuates or eliminates these changes in most patients.
Barbiturates depress the medullary ventilatory center, decreasing the ventilatory response to hypercapnia and hypoxia. Deep barbiturate sedation often leads to upper airway obstruction; apnea often follows an induction dose. During awakening, tidal volume and respiratory rate are decreased following barbiturate induction. Barbiturates incompletely depress airway reflex responses to laryngoscopy and intubation, and airway instrumentation may lead to bronchospasm (in asthmatic patients) or laryngospasm in lightly anesthetized patients.
 Barbiturates constrict the cerebral vasculature, causing a decrease in cerebral blood flow, cerebral blood volume, and intracranial pressure. Intracranial pressure decreases to a greater extent than arterial blood pressure, so cerebral perfusion pressure (CPP) usually increases. (CPP equals cerebral artery pressure minus the greater of jugular venous pressure or intracranial pressure.) Barbiturates induce a greater decline in cerebral oxygen consumption (up to 50% of normal) than in cerebral blood flow; therefore the decline in cerebral blood flow is not detrimental. Barbiturate-induced reductions in oxygen requirements and cerebral metabolic activity are mirrored by changes in the electroencephalogram (EEG), which progress from low-voltage fast activity with small doses to high-voltage slow activity, burst suppression, and electrical silence with larger doses. Barbiturates may protect the brain from transient episodes of focal ischemia (eg, cerebral embolism) but probably do not protect from global ischemia (eg, cardiac arrest). Abundant animal data document these effects but the clinical data are sparse and inconsistent. Furthermore, thiopental doses required to maintain EEG suppression (most often burst suppression or flat line) are associated with prolonged awakening, delayed extubation, and the need for inotropic support.
Barbiturates constrict the cerebral vasculature, causing a decrease in cerebral blood flow, cerebral blood volume, and intracranial pressure. Intracranial pressure decreases to a greater extent than arterial blood pressure, so cerebral perfusion pressure (CPP) usually increases. (CPP equals cerebral artery pressure minus the greater of jugular venous pressure or intracranial pressure.) Barbiturates induce a greater decline in cerebral oxygen consumption (up to 50% of normal) than in cerebral blood flow; therefore the decline in cerebral blood flow is not detrimental. Barbiturate-induced reductions in oxygen requirements and cerebral metabolic activity are mirrored by changes in the electroencephalogram (EEG), which progress from low-voltage fast activity with small doses to high-voltage slow activity, burst suppression, and electrical silence with larger doses. Barbiturates may protect the brain from transient episodes of focal ischemia (eg, cerebral embolism) but probably do not protect from global ischemia (eg, cardiac arrest). Abundant animal data document these effects but the clinical data are sparse and inconsistent. Furthermore, thiopental doses required to maintain EEG suppression (most often burst suppression or flat line) are associated with prolonged awakening, delayed extubation, and the need for inotropic support.
The degree of central nervous system depression induced by barbiturates ranges from mild sedation to unconsciousness, depending on the dose administered (Table 9-1). Some patients relate a taste sensation of garlic, onions, or pizza during induction with thiopental. Barbiturates do not impair the perception of pain. In fact, they sometimes appear to lower the pain threshold. Small doses occasionally cause a state of excitement and disorientation that can be disconcerting when sedation is the objective. Barbiturates do not produce muscle relaxation, and some induce involuntary skeletal muscle contractions (eg, methohexital). Relatively small doses of thiopental (50-100 mg intravenously) rapidly (but temporarily) control most grand mal seizures. Unfortunately, acute tolerance and physiological dependence on the sedative effect of barbiturates develop quickly.
| Agent | Use | Route1 | Concentration (%) | Dose (mg/kg) |
|---|---|---|---|---|
| Thiopental, thiamylal | Induction | IV | 2.5 | 3-6 |
| Methohexital | Induction
| IV
| 1
| 1-2
|
| Secobarbital, pentobarbital | Premedication | Oral
| 5 | 2-42
|
Barbiturates reduce renal blood flow and glomerular filtration rate in proportion to the fall in blood pressure.
Hepatic blood flow is decreased. Chronic exposure to barbiturates has opposing effects on drug biotransformation. Induction of hepatic enzymes increases the rate of metabolism of some drugs, whereas binding of barbiturates to the cytochrome P-450 enzyme system interferes with the biotransformation of other drugs (eg, tricyclic antidepressants). Barbiturates promote aminolevulinic acid synthetase, which stimulates the formation of porphyrin (an intermediary in heme synthesis). This may precipitate acute intermittent porphyria or variegate porphyria in susceptible individuals.
Anaphylactic or anaphylactoid allergic reactions are rare. Sulfur-containing thiobarbiturates evoke mast cell histamine release in vitro, whereas oxybarbiturates do not. For this reason, some anesthesiologists prefer induction agents other than thiopental or thiamylal in asthmatic or atopic patients, but the evidence for this choice is sparse. There is no question that airway instrumentation with light anesthesia is troublesome in patients with reactive airways.
Contrast media, sulfonamides, and other drugs that occupy the same protein-binding sites as thiopental may displace the barbiturate, increasing the amount of free drug available and potentiating the organ system effects of a given dose.
Ethanol, opioids, antihistamines, and other central nervous system depressants potentiate the sedative effects of barbiturates. The common clinical impression that chronic alcohol abuse is associated with increased thiopental requirements during induction lacks scientific proof.
Benzodiazepines
Benzodiazepines bind the same set of receptors in the central nervous system as barbiturates but bind to a different site on the receptors. Benzodiazepine binding to the GABAA receptor increases the frequency of openings of the associated chloride ion channel. For example, benzodiazepine-receptor binding facilitates binding of GABA to its receptor. Flumazenil (an imidazobenzodiazepine) is a specific benzodiazepine-receptor antagonist that effectively reverses most of the central nervous system effects of benzodiazepines (see Chapter 17).
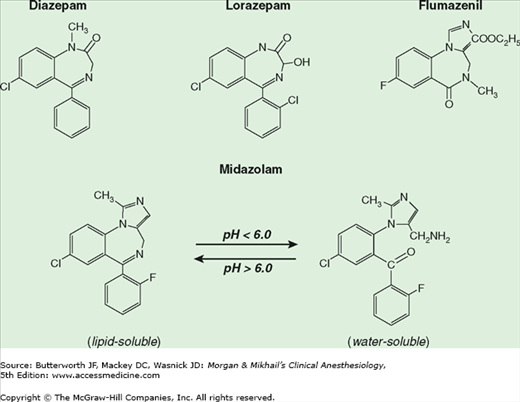
The chemical structure of benzodiazepines includes a benzene ring and a seven-member diazepine ring (Figure 9-3). Substitutions at various positions on these rings affect potency and biotransformation. The imidazole ring of midazolam contributes to its water solubility at low pH. Diazepam and lorazepam are insoluble in water so parenteral preparations contain propylene glycol, which can produce venous irritation.
Figure 9-3
The structures of commonly used benzodiazepines and their antagonist, flumazenil, share a seven-member diazepine ring. (Modified and reproduced, with permission, from White PF: Pharmacologic and clinical aspects of preoperative medication. Anesth Analg 1986;65:963. With permission from the International Anesthesia Research Society.)
Benzodiazepines are commonly administered orally, intramuscularly, and intravenously to provide sedation or, less commonly, to induce general anesthesia (Table 9-2). Diazepam and lorazepam are well absorbed from the gastrointestinal tract, with peak plasma levels usually achieved in 1 and 2 h, respectively. Oral midazolam has not been approved by the U.S. Food and Drug Administration, nevertheless this route of administration has been popular for pediatric premedication. Likewise, intranasal (0.2-0.3 mg/kg), buccal (0.07 mg/kg), and sublingual (0.1 mg/kg) midazolam provide effective preoperative sedation.
| Agent | Use | Route1 | Dose (mg/kg) |
|---|---|---|---|
| Diazepam | Premedication | Oral | 0.2-0.52 |
| Sedation | IV | 0.04-0.2 | |
| Midazolam | Premedication | IM | 0.07-0.15 |
| Sedation | IV | 0.01-0.1 | |
| Induction | IV |