Fig. 16.1
A CT scan of human chest showing the close spatial relationship between the heart and the lungs
The heart and lung (H-L) interaction can be defined as the effects of airway pressure and volume on venous return, ventricular function and arterial outflow [1]. Such effects are summarised in Fig. 16.2.
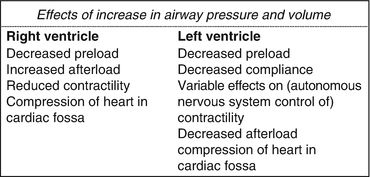

Fig. 16.2
Effects of increase in airway pressure and volume on the heart
H-L interactions are different in healthy and diseased conditions and between spontaneous and positive pressure ventilation.
Under normal conditions and in spontaneous ventilation, the H-L interaction is subtle. In the presence of cardiac and respiratory disease states, or when the compensatory reserve is limited, such as in critically ill patients, and under positive pressure ventilation (either invasive either non-invasive), H-L interactions are elicited, and the cardiovascular function can be affected by respiratory changes.
As ARDS (Acute Respiratory Distress Syndrome) pathology involves both alveoli and pulmonary capillaries, as reported long ago [2], it represents a typical predisposing situation for the H-L interaction to influence the circulation.
16.2 H-L Interactions: The Impact of Cardiorespiratory System Characteristics
The description of the anatomy and function of the heart and lungs is basic knowledge that is beyond the scope of this chapter. Therefore, only some key aspects of the cardiorespiratory system relevant to the H-L interaction issue of the ARDS patient will be addressed here.
The human heart shows the interesting combination of physiologically consisting of a double pump system where the two pumps, the left and right ventricles, work in series, with each pump ejecting the blood received from the other pump, and anatomically consisting of two pumps sharing the interventricular septum and the pericardium, thus being anatomically in parallel (Fig. 16.3). One more important aspect to underline in this contest is the anatomic and functional presence of the pulmonary circulation between the two pumps within the chest. This peculiar anatomic and functional condition of two pumps structurally made in parallel and mechanically working in series, with the pulmonary circulation being the circuit connecting the right ventricular ejection to the left ventricle, represents the main reason for the H-L interactions to occur and to have consequences in diseased states, and it is pivotal to understanding the ventricular interdependence. In fact, any acute and relevant change of the anatomy and/or the function of one pump or of the pulmonary circulation will cause the H-L interactions to be clinically significant.
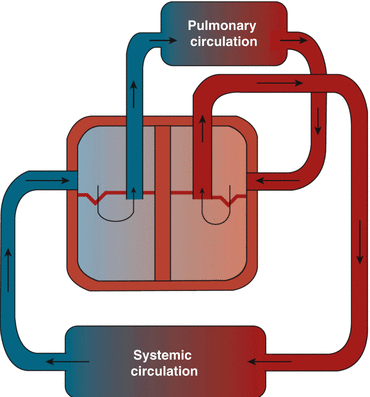

Fig. 16.3
Pulmonary and systemic circulation (to modify) (Modified with permission from F. Guarracino “Ecocardiografia in Area critica” 2013 Elsevier, Milan)
In the setting of ARDS, it is the relationship between the right ventricle (RV) and the pulmonary circulation that plays a major role, in a setting where the physiological interplay of the pump and vascular elastances is pivotal in determining the right ventriculo-arterial coupling.
It is wise to distinguish H-L interactions under spontaneous ventilation from those observed under positive pressure ventilation.
16.3 H-L Interactions in Spontaneous Ventilation
Heart-lung interactions occur at every breath. Under normal conditions, the changes in pressure and volume within the lungs (Fig. 16.4) will have no relevant haemodynamic effect. However, if a respiratory or cardiac disease is present, either acute or chronic, then the normal respiratory physiology is altered, and this may have circulatory consequences.
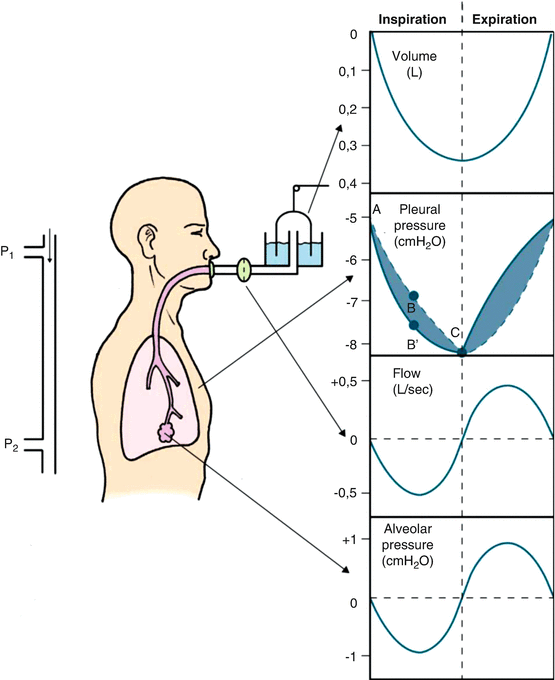

Fig. 16.4
Changes of airways pressure, flow and volume and of pleural pressure during spontaneous ventilation (Modified with permission from F. Guarracino “Ecocardiografia in Area critica” 2013 Elsevier, Milan)
In the setting of acute dyspnoea, such as in asthma, or in the acute exacerbation of a chronic respiratory disease, the increased swing in pleural pressure leads to an increasingly negative intrathoracic pressure during inspiration [3], which increases the venous return. This increased venous return increases the right ventricular volume, causing the intraventricular septum to move into the left ventricle. This leads to a decreased left ventricular diastolic compliance and a decrease in left ventricular filling volume during the inspiratory phase. As a consequence, the stroke volume and pulse pressure are reduced, and the features of pulsus paradoxus appear.
Therefore, during dyspnoea under spontaneous ventilation, the greater the pleural pressure, the greater the pulsus paradoxus.
This increased negative shift in intrathoracic pressure is not only responsible for the pulsus paradoxus but also causes acute changes in the cardiac transmural pressure, with increased myocardial oxygen consumption, reduced cardiac output and reduced flow to the respiratory muscles. The consequences of this H-L interaction will be haemodynamic impairment and further deterioration of the respiratory state.
An acute hypoxaemia from any respiratory cause will lead to pulmonary vasoconstriction and an increased RV afterload. The consequence will be an increased RV afterload and an RV/pulmonary circulation uncoupling.
Acute changes in the pulmonary circulation, such as in pulmonary embolism or in pulmonary oedema, will also affect H-L interactions and cause RV/pulmonary circulation uncoupling through an increased afterload to the RV [4]. The effect will be a reduced systemic flow and, again, an acute increase in the myocardial transmural pressure via the increased negativisation of inspiratory pleural pressure.
In some of the described acute disease states, the treatment includes positive pressure ventilation, which contributes to further elicit the H-L interactions.
16.4 H-L Interactions in Positive Pressure Ventilation
When a patient is under positive pressure ventilation (PPV), the interaction between the lungs and the heart is elicited, and haemodynamic effects can be observed [1, 5].
The effects of airway pressure and volume can be addressed separately.
The inspiratory positive pressure is transmitted to the right atrium (RA) and vena cava and below the diaphragm to the abdominal district. A direct compression of the great veins in the cardiac fossa and of the RA wall by the lungs must also be taken into account. This process causes a reduction in the venous return to the right heart [6] due to an increase in the difference between the transpulmonary pressure (TP) and filling pressure, which represents the gradient for the venous return. Such a reduction in the RV preload causes a reduction in the RV stroke volume and then a reduction in the cardiac output.
A specific aspect related to the effects of ventilatory pressure in the setting of mechanical ventilation in ARDS patients regards the decreased lung compliance that such a disease carries [7, 8]. In fact, a reduction in lung compliance leads to a reduced transmission of inspiratory positive pressure, with potentially fewer haemodynamic effects. However, if a patient with ARDS is kept at a constant tidal volume (TV), then his/her airway pressure will be increased regardless if compared to baseline healthy conditions; thus, both the intrathoracic pressure and the right heart pressure will increase and cause a reduction in the venous return. Therefore, despite a reduction in lung compliance in ARDS, the haemodynamic consequences of H-L interactions under mechanical positive pressure ventilation cannot be excluded.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





