153 Inhaled Toxins
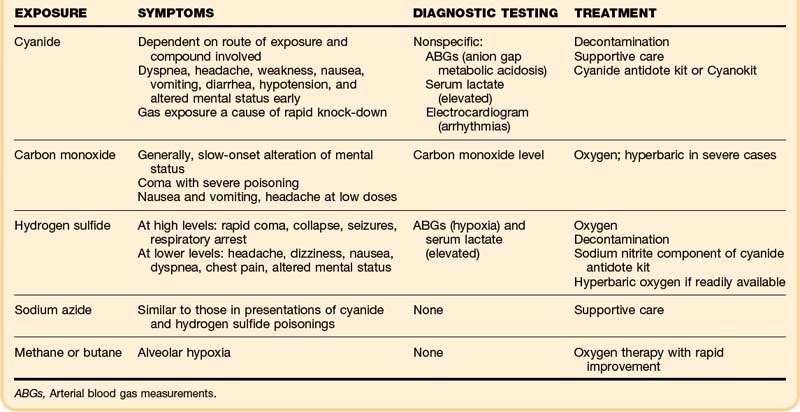
• Cyanide gas and hydrogen sulfide are rapid knockdown agents.
• The key characteristic of cyanide is profound metabolic acidosis with a wide anion gap and elevation of serum lactate.
• Clinical suspicion is paramount to the diagnosis of cyanide toxicity; treatment may be required before the diagnosis is confirmed.
• Hydroxycobalamin (Cyanokit) has fewer adverse events than the cyanide kit; it has one component instead of three.
• Rescuers must wear adequate personal protective equipment, including a self-contained breathing apparatus, to remove a victim from the source of exposure to hydrogen sulfide.
• Patients who present alert, without altered mental status and with normal respiratory effort after exposure to hydrogen sulfide, should have a good outcome.
• If administered shortly after severe hydrogen sulfide exposures, sodium nitrite may improve outcome.
• The mainstays of treatment for hydrogen sulfide toxicity are 100% oxygen and meticulous supportive care.
Cyanide
Epidemiology
Cyanide exists in several forms. The gaseous form is hydrogen cyanide (HCN); the salt forms are potassium cyanide and sodium cyanide. The inorganic salts release HCN gas when they are dissolved in water. Cyanogens are compounds that are metabolized to cyanide in vivo. The two clinically important cyanogens are amygdalin and acetonitrile. Amygdalin is a naturally occurring cyanogen found in the seeds of plants in the Prunus species and in the pits of other fruits (Box 153.1). Acetonitrile is found in some artificial nail removal products. Unlike other cyanide products, cyanogens may produce delayed cyanide toxicity because of the time required for their biotransformation. Cyanide gas is also released during combustion of certain natural and synthetic materials (Box 153.2). Consequently, cyanide poisoning can occur in victims of structure fires.
Box 153.1
Cyanogen-Containing Plants
From Hall AH, Rumack BH. Clinical toxicology of cyanide. Ann Emerg Med 1986;15:1067–74.
Pathophysiology
Cyanide is a potent cellular poison. The primary clinical effect occurs through the inhibition of oxidative phosphorylation. Cyanide binds to the ferric (Fe3+) moiety of cytochrome oxidase aa3, the last enzyme of the electron transport chain.1 The net effects of cyanide poisoning are reduced oxygen consumption and conversion of aerobic to anaerobic metabolism; these effects lead to profound metabolic acidosis and an elevated serum lactate concentration.
Differential Diagnosis and Medical Decision Making
Without a history of cyanide exposure, the diagnosis is often difficult to make (Table 153.1). All medical causes of altered mental status, hypotension, and acidosis must be considered. The differential diagnosis of cyanide poisoning is listed in Box 153.3.
Box 153.3 Differential Diagnosis of Cyanide and Hydrogen Sulfide Poisoning
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree