138 Infectious Endocarditis
Infectious endocarditis (IE) is a rare disease with an incidence of 3 to 10 episodes per 100,000 person-years, varying between countries and increasing dramatically with age. It is presently classified by mode of acquisition (healthcare-associated IE [nosocomial and non-nosocomial], community acquired, and IE in intravenous drug users [IVDU]), by localization as left- or right-sided prosthetic or native valve IE, or as device related (e.g., pacemaker or cardioverter defibrillator). The new classification of healthcare-associated infectious endocarditis (HAIE) includes patients hospitalized for more than 48 hours before symptoms of IE develop (previously called nosocomial IE [NIE]) or patients with symptoms less than 48 hours after admission but with extensive healthcare contact defined as: (1) home-based nursing or IV therapy, hemodialysis, or IV chemotherapy fewer than 30 days before onset of IE symptoms, (2) hospitalization fewer than 90 days before onset of IE, or (3) residency in a nursing home or a long-term care facility. The definition of HAIE applies both to native (NVE) and prosthetic valve endocarditis (PVE). Early prosthetic valve endocarditis (now defined as presenting <1 year post surgery) has a portion included in the HAIE definition.1
HAIE was estimated to have occured in 0.8 of 10,000 hospital admissions and is often diagnosed late during hospitalization (39 ± 25 days).2 When compared with the 2.5 million cases (at least) of nosocomial infections occurring per year in the United States, the overall incidence of HAIE seems low,2 but the associated morbidity and high mortality renders HAIE of great importance for the clinician. The current in-hospital mortality rate for patients with IE is 15% to 20%, with 1-year mortality approaching 40%.3
 Healthcare-Associated Native Valve Endocarditis
Healthcare-Associated Native Valve Endocarditis
During the past decade, 14% to 25% of all cases of IE have been considered nosocomial. It is, however, expected that the incidence will increase in the future because of (1) an increase in the incidence of nosocomial bacteremia, (2) improvement in survival of immunocompromised patients, (3) the steady increase in the number of ICU beds admitting seriously ill patients worldwide, and (4) the improved survival rate of elderly patients in whom degenerative heart disease and/or prosthetic valves are more frequently encountered.2 The current understanding of HAIE has been based primarily on retrospective studies with small sample size. New data emerged from the International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) from 61 medical centers in 28 countries.4 From this database, as defined by the modified Duke criteria, native valve IE in patients without IV drug abuse was recognized in 1622 patients. Of these patients, 1065 had community-acquired infection, and 557 (34%) had healthcare-associated native valve endocarditis (HANVE), consistent with the contemporary high incidence of healthcare-associated infection.5 Almost half of these infections were acquired outside of the hospital, a result consistent with previous reports of healthcare-associated bacteremia. Compared with patients with community-acquired IE, patients with HANVE more often have comorbid conditions such as diabetes mellitus, cancer, or long-term immunosuppressive therapy. Fever is the most common presenting feature, but physical signs of IE present more rarely in HANVE, suggesting a more acute course. Non-nosocomial acquisition of HANVE is most often dependent on hemodialysis or an intravascular catheter (54%), while patients with nosocomial acquisition more often have preexisting valvular disease or undergo a non-dental invasive medical procedure. The mitral valve is most frequently involved, followed by the tricuspid and aortic valve.4
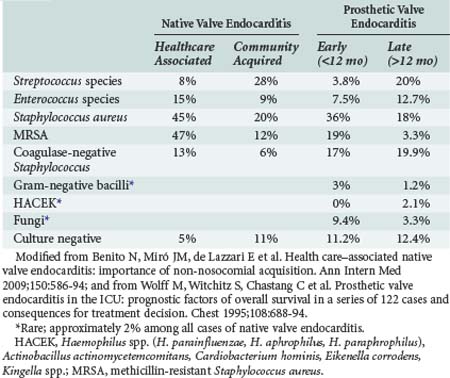
Staphylococci (both Staphylococcus aureus and coagulase-negative strains) represent the major pathogens in HAIE. S. aureus is responsible for 52% to 57% of HAIE episodes, 91% of which have an intravascular device as the most probable source of bacteremia.2 In the ICE-PCS study, S. aureus was the most common pathogen in HANVE, among which 47% was methicillin-resistant S. aureus (MRSA).4 The second most common bacteria was enterococci (15%), followed by coagulase-negative strains of staphylococci (13%). MRSA is more prevalent in hospital-acquired infections (57% versus 41% of HANVE acquired outside the hospital).4 Among coagulase-negative strains of staphylococci, Staphylococcus lugdunensis deserves attention because it behaves like S. aureus with high virulence, has a 50% probability of complicated infection when isolated in blood, and an aggressive course when it is the cause of IE.6
Gram-negative bacilli are rare causes of HANVE despite the fact that they cause lethal bacteremias in hospitals, probably as a result of their decreased ability to adhere to heart valves and susceptibility to bactericidal action of serum.2,7 Fungal infectious endocarditis is a rare infection, comprising in total less than 10% of IE cases, with a mortality rate ranging from 36% to 50%. However, increased frequency of fungal endocarditis has been observed in recent years, attributed to the increasing use of vascular lines, as well as to noncardiac surgery and increased numbers of immunocompromised patients.8 The fungi most commonly associated with endocarditis are Candida albicans, non-albicans species of Candida, Aspergillus spp., and Histoplasma capsulatum. In the past decade, the incidence of Candida parapsilosis HAIE has increased and is attributed to (1) frequent colonization by this organism of the skin and subungual area, (2) ability of the pathogen to proliferate in glucose-containing solutions (hyperalimentation), (3) ability of the organism to adhere to synthetic material because of slime production, and (4) contamination of intravascular pressure monitoring devices.2
In contradistinction to Candida spp., in which blood cultures in cases of IE are positive in 83% to 95% of cases, blood cultures are positive in only 11% or less of patients with Aspergillus spp. In cases of Curvularia, Penicillium, and Phycomyces infection, blood cultures are usually 100% negative. In cases in which Coccidioides immitis, Cryptococcus neoformans, Rhodotorula, and Saccharomyces cerevisiae are involved, blood cultures are usually positive if properly collected.2,8
In cases of fungal endocarditis, prolonged symptoms before hospitalization and embolization of major arteries are classic findings. However, diagnosis is delayed or missed in 82% of patients.8,9 For fungal endocarditis to be diagnosed early, it should be considered in the differential diagnosis and echocardiography performed, which then demonstrates large, bulky vegetations. Peripheral blood cultures should be obtained and accessible embolic specimens subjected to histologic examination.9,10
HANVE has higher mortality compared to community-acquired IE (25% versus 13%). In HANVE, factors recognized to be independently associated with increased risk of death are increased age (>60), diabetes, S. aureus infection, paravalvular abscess, stroke, heart failure, and new conduction abnormality. Cardiac surgery during the IE episode is found to be associated with lower mortality.4 Therefore, in addition to appropriate antimicrobial therapy, early surgical intervention is often mandatory. In fungal endocarditis, removal of the infected valve is indicated, followed by postsurgical prophylaxis with oral azoles for 2 or more years and prolonged surveillance to detect relapses.9,10
Special consideration should be given to chronic hemodialysis (HD) patients, in whom IE is significantly more common (16-18 times) and causes greater morbidity and mortality. In this group of patients, IE is the second leading cause of death after cardiovascular disease, and it has been proposed to be added as a fifth category in classification by acquisition.11,12 In the ICE-PCS study, 63% of HANVE were HD patients.4 S. aureus was the pathogen in 75% to 80% of cases, half of which were MRSA. Fever may not be present, and blood cultures may less often be positive, complicating diagnosis by the Duke criteria. Mortality remains high: 30% during the first month, about 65% during the first year, and reaching more than 70% if cardiac surgery is indicated. Age older than 65, diabetes as the cause of renal failure, mitral involvement, large vegetations, septic emboli, and infections due to MRSA or VRE have been identified as risk factors for mortality.11
For methicillin-sensitive S. aureus (MSSA), antistaphylococcal penicillins should be the treatment of choice, whereas in cases of MRSA with minimum inhibitory concentration (MIC) over 1 mg/L to vancomycin, antimicrobial choices include daptomycin and linezolid.1 If vancomycin is indicated, drug levels should be followed, with trough levels of 25 to 30 mg/L required for efficacy.13
 Healthcare-Associated Prosthetic Valve Endocarditis
Healthcare-Associated Prosthetic Valve Endocarditis
PVE accounts for 9.5% to 20% of all cases of IE, with mortality rates ranging between 25% and 60%.14 It is a distinct and important form of IE because more than 100,000 artificial heart valves are implanted annually in the United States, and eradicating infection on foreign material is a major therapeutic challenge which as a rule necessitates their surgical removal.14
It has been reported that “early” PVE (within 1 year of implantation) is found less often with porcine than mechanical valves, whereas it is almost absent from homografts. However, studies with long-term follow-up have suggested that no significant differences exist in the incidence of PVE related to the valve type.14 As noted, PVE has been classified as early or late, with the former occurring within 12 months of implantation.1 Contamination of prosthetic valves during this early period occurs either directly at the time of implantation by a break in sterile surgical techniques or via transient episodes of bacteremia, emanating mostly from infected intravascular catheters and wound or skin infections while the patient is still hospitalized, therefore representing a real nosocomial infection.14 In the early postoperative period, the sewn ring and the valve annulus are not yet endothelialized and are therefore a site of thrombus formation and a target for adherence of bacteria. Transient bacteremia can seed these thrombi and incite infection, leading to the formation of large vegetations that may cause functional obstruction or incompetence. As the infection advances, abscesses, fistulas, and progressive annular destruction may further complicate the underlying process, causing conduction blocks, mycotic aortic aneurysms, and even purulent pericarditis.14
PVE may manifest as an indolent illness with low-grade fever and immune-mediated manifestations or as a fulminant acute febrile disease with hypotension. When early PVE is caused by S. aureus, the clinical picture is accompanied in more than 40% of cases by central nervous system (CNS) and intracardiac complications and a subsequent mortality ranging from 42% to 85%.15 The microbiology of PVE is shown in Table 138-1. In the ICE-PCS study, 556 definite cases of PVE were found among 2670 cases of IE (20%), with 36.5% being healthcare-associated prosthetic valve endocarditis (HAPVE) and 70% acquired in the hospital.16 Of the cases of PVE, 71% were diagnosed during the first year post surgery and the majority after day 60 (median on day 84). In 43% of HAPVE, an intravascular device was in place. S. aureus was the most common pathogen in PVE, with higher incidence in cases with HAPVE (34% and 13.3% MRSA), followed by coagulase-negative staphylococci.16
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree