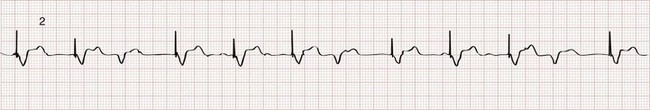
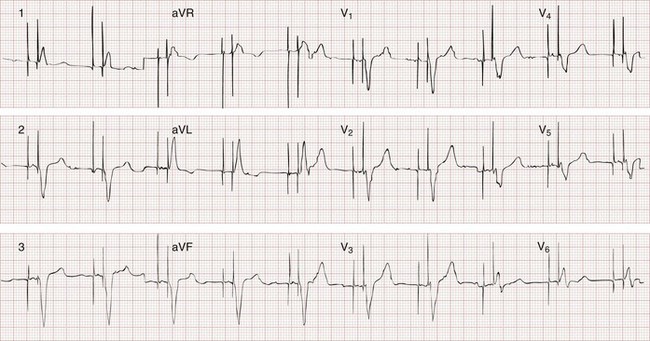
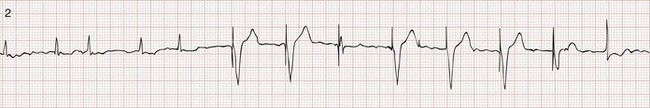
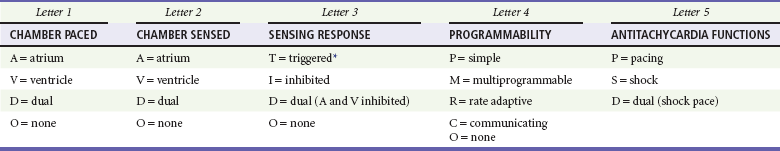
Chapter 80 Electrical cardiac pacing for the management of bradyarrhythmias was first described in 1952, and permanent transvenous pacing devices were introduced into clinical practice in the early 1960s.1 The first devices for endocardial defibrillation were implanted in surviving victims of sudden cardiac death in 1980.2 Implanted electrical devices for the management of cardiac dysrhythmias have changed rapidly over the years, with both increasing complexity and miniaturization. Survey data from 2002 indicated that approximately 612 new pacemakers per million population were implanted in the United States.3 Indications for the use of permanent pacemakers in the management of congenital and acquired heart disease include cardiac resynchronization therapy for heart failure.4–6 A number of large clinical trials comparing implantable cardioverter-defibrillators (ICDs) with antiarrhythmic drugs for the prevention of sudden cardiac death resulting from ventricular dysrhythmias have indicated that ICDs significantly improve survival.7,8 Such studies have led to a dramatic increase in ICD implantations, and it is estimated that there are more than 125,000 new ICD implants annually in the United States.9 The widespread use of these devices ensures that the emergency physician frequently encounters such patients, often with symptoms that may be related to malfunction of the pacemaker or ICD. Guidelines for the implantation of these devices have been developed by a joint task force of the American Heart Association (AHA) and the American College of Cardiology (ACC) and are periodically updated.10 With use of an evidence-based approach, recommendations are categorized as class I, II, or III. Class I includes conditions for which there is general agreement that a device should be implanted. A class II recommendation includes conditions for which these devices are frequently used but for which there is disagreement about their need or benefit. Class III is reserved for conditions for which there is general agreement that a device is not needed. In the case of pacemaker therapy, additional factors are considered when selecting the mode of pacing and include, but are not limited to, overall health, lifestyle, and occupation of the patient. Class I indications for a permanent pacemaker or ICD are listed in Boxes 80-1 and 80-2. In general, pacing is recommended for patients with symptomatic heart block, symptomatic sinus bradycardia, and atrial fibrillation with a symptomatic bradycardia (low ventricular response rate) in the absence of medications that affect atrioventricular (AV) conduction. Controversial indications include pacing in patients with syncope, heart block, or fatigue in the presence of some conduction disease or bradycardia. The likelihood of a patient’s improvement after pacing can be assumed only if the symptoms can be closely correlated with inadequate rate. A letter code, initially established in 1974 and revised as technology advances, standardizes nomenclature for pacemakers.11 Table 80-1 includes an explanation of the five-letter code scheme and the standard abbreviations in each category. The first three code letters are used most commonly. Using this table, one should be able to understand the features of any pacing mode. For example, a VDD pacemaker is capable of pacing only the ventricle, sensing both atrial and ventricular intrinsic depolarization, and responding by dual inhibition of both atrial and ventricular pacing if intrinsic ventricular depolarization occurs; a paced ventricular beat is triggered in response to a sensed intrinsic atrial depolarization. The codes of a permanent pacemaker that are used most frequently and the indications, advantages, and disadvantages of each are listed in Table 80-2. Detailed algorithms for matching a patient with a pacemaker exist.10 The majority of permanent pacemakers are dual chamber and often rate adaptive.12 Table 80-1 *In the triggered response mode, the pacemaker discharges or fires when it recognizes an intrinsic depolarization. As a result, pacemaker spikes occur during inscription of the QRS complex. Because this mode results in high energy consumption and a shortened battery life and because the sensing response can be misinterpreted as pacemaker malfunction, this sensing mode is not used with modern pacemakers. Permanent pacemakers have endocardial leads that are positioned in contact with the endocardium of the right ventricle and, in the case of a dual-chamber device, the right atrium, with a subclavian or cephalic vein approach used for insertion. Occasionally, an epicardial lead may be implanted during open-heart surgery performed for another indication, such as prosthetic valve insertion or correction of a congenital cardiac defect. Pacemaker leads, like power sources, continue to undergo major technical improvements.1 Innovations include resilient plastic insulation surrounding the electrodes that reduces the chance of complete lead disruption or breakage (resulting in failure to pace or sense) and the chance of partial fracture (resulting in a “make or break” contact with intermittent failure to sense or pace). Despite these advances, problems with the electrical circuitry remain the most common cause of pacemaker malfunction.13 A lead capable of active fixation is more commonly used in patients with cardiomyopathies and right ventricular dilation complicated by tricuspid regurgitation. Pacemaker leads may be either bipolar or unipolar in configuration. A bipolar endocardial lead has both the negative (distal) and the positive (proximal) electrodes, separated by approximately 1 cm, within the heart. A unipolar lead has the negative electrode in contact with the endocardial surface, and the positive pole is the metallic casing of the pulse generator. Each lead system has potential advantages and disadvantages.1 The unipolar configuration is not compatible with ICD systems and is prone to oversensing of myopotentials and electromagnetic interference but is of smaller diameter and less susceptible to fracture. The bipolar configuration is compatible with ICD systems but is larger and more prone to lead fractures. Oversensing, however, is rarely a problem. The selection of lead configuration usually depends on the experience and preference of the operator. The modern pacemaker has two basic functions: to stimulate the heart electrically and to sense intrinsic cardiac electrical activity. Additional functions are available and are noted in the pacemaker code system (see Table 80-1, letters 4 and 5). The pacemaker delivers an electrical stimulus to either the atrium or the ventricle if it does not recognize (sense) any intrinsic electrical activity from that chamber after a selected time interval. This interval is usually programmed at the time of implantation and can be changed noninvasively at a later time, if necessary, with use of a programming and “interrogating” device provided by the pacemaker manufacturer. If the pacemaker recognizes or senses an intrinsic atrial depolarization (P wave) or ventricular depolarization (QRS complex), it inhibits or resets its output to prevent competition with the underlying intrinsic rhythm. The stimulus intensity and sensing threshold (amplitude of electrical activity that is detected as being intrinsic) are typically set at the time of implantation but can also be reprogrammed later. The two basic functions of a pacemaker can be easily recognized and confirmed on a standard 12-lead electrocardiogram (ECG) or rhythm strip. The normal function of a single-chamber VVI pacemaker is most easily recognized (Fig. 80-1). After a programmed interval is surpassed during which intrinsic ventricular activity does not occur, a pacer “spike” or stimulus artifact appears. The pacer spike is a narrow deflection that is usually less than 5 mm in amplitude with a bipolar lead configuration and usually 20 mm or more in amplitude with a unipolar lead. A wide QRS complex appears immediately after the stimulus artifact. Depolarization begins in the right ventricular apex, and the spread of excitation does not follow normal conduction pathways. Characteristically, a left bundle branch block conduction pattern is seen. A right bundle branch pattern is abnormal and suggests lead displacement. In VVI pacing the paced QRS complexes are independent of intrinsic atrial depolarization if present (AV dissociation). The recognition of normal dual-chamber pacing is more complex owing to the interactive sensing and pacing of the right atrium and ventricle (Fig. 80-2).14 Pacing intervals are preprogrammed, may be changed noninvasively at a later time, and are generally specific to the patient’s needs. Pacing rates and delay intervals typically vary from patient to patient. Dual-chamber devices are typically used in patients with nonfibrillating atria coupled with intact AV conduction. A normal-appearing QRS complex may follow an intrinsic “P” wave as a result of normal sinoatrial node discharge if the intrinsic atrial depolarization is conducted to the ventricles. The intrinsic p wave and QRS complex inhibit the atrial and ventricular circuitry. A normal QRS complex follows a paced p wave if the paced atrial beat is conducted through the AV node and the programmed AV delay period is not exceeded. If it is not conducted to the ventricles (AV delay period exceeded), the pacemaker stimulates the ventricle, resulting in a paced p wave and a wide, paced QRS complex with left bundle branch block configuration. Recognition of the interactivity of the paced chambers is important. A paced p wave may be mistaken for failure to sense or pace, and malfunction may be diagnosed when it is not present (pseudomalfunction). In addition, if the programmed rate of the pacemaker approximates the patient’s intrinsic heart rate, fusion of paced and native beats may occur and represents another common type of pseudomalfunction (Fig. 80-3). Pacemaker implantation is a surgical procedure and, like all surgery, carries a risk of infection; the presence of a foreign body enhances this risk. The incidence of infection is small—approximately 2% for wound and subcutaneous pacemaker “pocket” infection and approximately 1% for bacteremia with sepsis. The presence of a foreign body complicates management, and few cases of bacteremia that develop after implantation can be managed with antibiotics alone. In most instances, reimplantation and replacement of the lead system are necessary.15 When a local infection or bacteremia is suspected, blood cultures should be obtained and intravenous antibiotic therapy initiated. Staphylococcus aureus and Staphylococcus epidermidis are isolated in approximately 60 to 70% of cases. Empirical antibiotic therapy should include vancomycin pending culture and sensitivity data. If blood cultures are positive, the pulse generator and pacemaker lead are usually removed, temporary transvenous pacing is performed, and intravenous antibiotic therapy is continued for 4 to 6 weeks. The permanent pacemaker and lead are subsequently reimplanted.15 The incidence of venous obstruction associated with permanent transvenous pacemakers ranges from 30 to 50%, with approximately one third of patients having complete venous occlusion.16 Thrombosis of varying degrees can involve the axillary, subclavian, and innominate veins or the superior vena cava (SVC). The site of insertion does not appear to affect the incidence of this complication. Chronic thrombosis of the veins of the upper arm is common and usually asymptomatic owing to extensive venous collateral circulation. These symptoms are termed the pacemaker syndrome.17 The cause of this syndrome is the loss of AV synchrony and the presence of ventriculoatrial conduction, and it is most commonly encountered in the setting of VVI pacing. It is also described with the DDI mode. With VVI pacing, the ventricle is electrically stimulated and depolarized, resulting in ventricular systole. If sinus node function is intact, the atria can be depolarized by a sinus impulse and contract when the tricuspid and mitral valves are closed. This contractile asynchrony results in an increase in jugular and pulmonary venous pressures and may produce symptoms of congestive heart failure. Approximately 20% of patients report symptoms suggesting the pacemaker syndrome after pacemaker insertion. In most instances, symptoms are mild and patients adapt to them. In approximately one third of these patients, symptoms are severe. Treatment usually requires replacing a VVI pacemaker with a dual-chamber pacemaker or lowering the pacing rate of the VVI unit. If symptoms occur in a patient paced in the DDI mode, optimizing the timing of atrial and ventricular pacing is usually required. Patients appear to prefer dual-chamber pacing to the VVI modality.18 Persistence or worsening of symptoms of congestive heart failure may also be caused by the altered ventricular activation sequence inherent with stimulation of the right ventricle.19 Biventricular pacing may be required in selected patients.
Implantable Cardiac Devices
Perspective
Indications for Permanent Pacemakers and Antiarrhythmia Devices
Pacemaker Terminology
Pacemaker Components
The Standard Electrocardiogram during Normal Cardiac Pacing
Complications of Implantation
Thrombophlebitis
The “Pacemaker Syndrome”
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Implantable Cardiac Devices
Only gold members can continue reading. Log In or Register to continue