CHAPTER 101 HYPOTHERMIA AND TRAUMA
Human beings, as homeotherms, normally maintain their body temperature within a narrow range around a core temperature of 37° C. A variety of built-in mechanisms work to either preserve or lose heat. The failure of these mechanisms can result in abnormal temperatures and associated pathophysiologic consequences.
Hypothermia, defined as a core temperature of 35° C or less, is a strong predictor of mortality after injury.1–3
INCIDENCE
A recent analysis of the National Trauma Data Bank (NTDB) provides the most comprehensive perspective on the incidence of hypothermia among trauma patients.4 Of 1,126,238 injured patients, the admission body temperature was recorded in 701,491 (62.3%). A total of 11,026 patients (1.57% of all patients with a recorded temperature) were hypothermic, defined as a core temperature lower than 35° C.
MECHANISM OF INJURY
Trauma patients are disrobed in the emergency department, where most heat loss occurs, and are frequently administered cold intravenous (IV) fluids. Hypothermia is more common and more profound in the more seriously injured patients. Therefore, there is uncertainty over whether the increase in mortality is primarily attributable to the hypothermia itself, or to the underlying injuries. Some have proposed that hypothermia is actually protective in trauma patients, and that mortality rates would not be higher in cold patients if comorbid factors were equal.5 However, recent studies have documented an adverse effect of hypothermia on outcome, and a significantly improved likelihood of surviving initial resuscitation when hypothermia is aggressively treated.1,3,4,6,7
EFFECTS ON COAGULATION
Perhaps the most serious effect of hypothermia in the trauma victim is its effect on coagulation. Uncontrollable hemorrhage, often compounded by coagulopathy, is the most frequent cause of early death in these patients. Dilutional thrombocytopenia is usually cited as the primary cause of coagulopathic bleeding when trauma victims undergo massive transfusion.8 However, a prospective, randomized, double-blind controlled clinical trial indicated that dilutional thrombocytopenia is relatively infrequent and that prophylactic administration of platelets was not beneficial.9 Consumptive coagulopathy appeared to be the more common problem associated with massive transfusion.
EFFECTS ON PLATELET COUNT AND FUNCTION
During development of hypothermic cardioplegia for cardiac surgery, there was a surge of research interest in the effects of hypothermia on coagulation in the late 1950s. Experimental studies in dogs at that time demonstrated a reversible thrombocytopenia associated with systemic hypothermia.10,11 However, the thrombocytopenia observed actually occurred at very deep levels of hypothermia, well below that typically seen in a trauma setting.11–15 Yoshihara et al.16 reported that platelet counts dropped by only 20%-30% at an esophageal core temperature of 30° C.
In contrast, levels of hypothermia commonly encountered in clinical practice have been shown to have a significant effect on platelet function. Platelets experience a reversible inhibition of their function under conditions of local or systemic hypothermia, mediated at least in part through the temperature dependence of thromboxane B2 by platelets.17 Thromboxane B2 is a potent vasoconstrictor and platelet aggregating agent. Valeri et al.17 demonstrated this when they induced systemic hypothermia to 32° C in baboons, but kept one forearm warm using heating lamps and a warming blanket. Simultaneous bleeding time measurements in the warm and cold arm were 2.4 and 5.8 minutes, respectively. The authors concluded that warming to restore wound temperature to normal should be tried before resorting to transfusion therapy with platelets and clotting factors when treating hypothermic patients with nonsurgical bleeding.
EFFECT ON CLOTTING FACTOR LEVELS AND FUNCTION
Several studies performed on humans undergoing hypothermic open-heart surgery failed to demonstrate significant alterations in clotting test times except at extreme degrees of hypothermia (i.e., <20° C).16,18–21 Yet, clinical experience suggests otherwise. Many patients with less severe degrees of hypothermia will have a serious coagulopathy that appears related to the presence of the hypothermia. This apparent inconsistency has been resolved by the realization that coagulation during mild hypothermia is disturbed more from enzymatic dysfunction than it is from altered clotting factor levels in blood. This explains the inability for the experimental data from the 1950s and 1960s to correlate with the clinical experience, as the clotting tests performed by the early experimenters were routinely performed at 37° C instead of at hypothermic temperatures.
In recent years, a number of studies have been performed wherein the clotting tests were performed at hypothermic temperatures. Bunker and Goldstein,18 in a study previously mentioned of controlled hypothermia in 10 patients, measured clotting tests at the hypothermic temperature of the patients as well as at 37° C. While they found no significant changes in clotting times when performed at 37° C, they state that “prolongation of the clotting times for all coagulation tests except whole blood clotting times was consistently observed when performed at the hypothermic temperatures.”
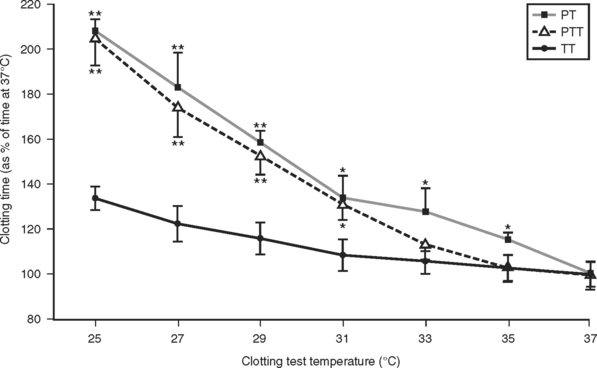
A detailed study of the kinetic effects of hypothermia on clotting factor function was undertaken by Reed et al.22 These studies were done by performing standardized clotting tests in a modified coagulation timer (fibrometer). Because the heat block of fibrometers used clinically are set by the manufacturer at 37° C, an external digital temperature controller was connected to the heat block power source to enable measurement of clotting times at the range of hypothermic temperatures typically encountered in trauma patients. Measurement of the prothrombin time, partial thromboplastin time, and thrombin time performed on assayed reference human plasma containing normal levels of all the clotting factors at temperatures ranging from 25° C to 37° C showed a significant slowing of clotting factor function that was proportional to the degree of hypothermia (Figure 1).
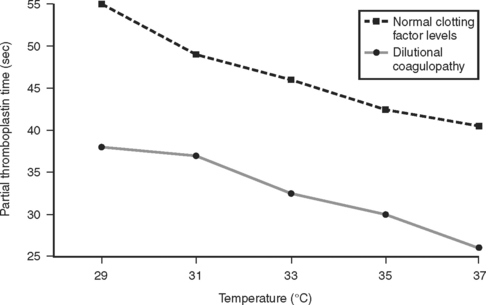
These results were later confirmed by Gubler et al.,23 in a study using a similar modified fibrometer that demonstrated an additive effect of hypothermia on dilutional coagulopathy (Figure 2).
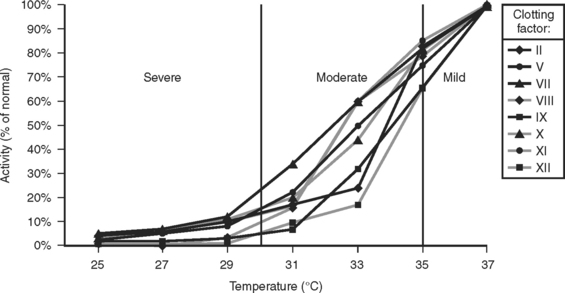
A subsequent study demonstrated that hypothermia could produce a coagulopathy functionally equivalent to a severe clotting factor deficiency, even at intermediate levels of hypothermia and even though there was no actual deficiency of clotting factors24 (Figure 3).
In summary, hypothermia does little to affect platelet and clotting factor levels, but it does a great deal to affect the function of these coagulation components. A recent analysis indicates that at mild temperature reductions between 33° C and 37° C, platelets are more profoundly affected than are clotting factors, although clotting factor dysfunction becomes increasingly severe as temperature cools further.25 Because of the potent effect that severe hypothermia has on platelet and clotting factor function, it is essential that body temperature be normalized before exogenous platelets or clotting factors are administered. Even though clotting studies may demonstrate severe clotting factor deficiencies, there is no value in transfusing coagulation components to severely hypothermic patients. This is because normal levels of clotting factors fail to clot effectively in the setting of severe hypothermia. Thus, administration of platelets or clotting factors to moderately or severely hypothermic patients is essentially futile, as the coagulation components will not function in a hypothermic environment (i.e., below 34° C).