BP during laryngoscopy (may result in transient hypotension).
• Requires intraarterial BP monitoring and use of infusion pump.
• Must be protected from light because of photodegradation.
Sodium Nitroprusside: Metabolism Key Points
• Thiocyanate is slowly cleared by the kidneys. In patients with renal failure, accumulation of large amounts of thiocyanate may result in thyroid dysfunction, muscle weakness, nausea, hypoxia, and acute toxic psychosis.
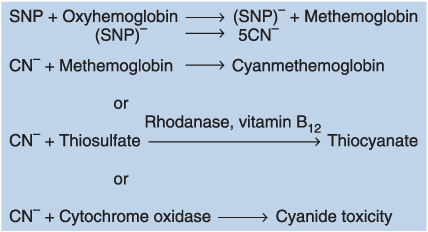
• The last of the three cyanide reactions is responsible for the development of acute cyanide toxicity, which is characterized by metabolic acidosis, cardiac arrhythmias, and increased venous oxygen content (inability to utilize oxygen).
• Another early sign of cyanide toxicity is the acute resistance to the hypotensive effects of escalating doses of sodium nitroprusside (tachyphylaxis).
• Cyanide toxicity can usually be avoided if cumulative dose of sodium nitroprusside is less than 0.5 mg/kg/h.
• Pharmacologic treatment of cyanide toxicity: Aim to shunt cyanide away from cytochrome oxidase.
° Sodium thiosulfate (150 mg/kg over 15 min)
° 3% sodium nitrate (5 mg/kg over 5 min): Oxidizes hemoglobin to methemoglobin
° Hydroxocobalamin: Combines with cyanide to form cyanocobalamin (vitamin B12)
• Methemoglobinemia from excessive doses of sodium nitroprusside or sodium nitrate can be treated with methylene blue (1–2 mg/kg of a 1% solution over 5 min); reduces methemoglobin to hemoglobin.
Sodium Nitroprusside: Effect on Organ Systems
• Combined dilation of venous and arteriolar vascular beds = ![]() preload and
preload and ![]() afterload =
afterload = ![]() BP.
BP.
Cardiac: Cardiac output is generally unchanged in normal patients.
• Cardiac output is increased in patients with congestive heart failure (CHF), mitral regurgitation, and aortic regurgitation secondary to ![]() afterload.
afterload.
• ![]() Preload,
Preload, ![]() myocardial work, and therefore
myocardial work, and therefore ![]() likelihood of ischemia.
likelihood of ischemia.
• Reflex-mediated tachycardia and contractility (offset the favorable changes in myocardial oxygen requirements).
• Dilation of coronary arterioles by sodium nitroprusside may result in an intracoronary steal of blood away from ischemic areas that are already maximally dilated.
Cerebral: Dilates cerebral blood vessels and abolishes cerebral autoregulation.
• Cerebral blood flow (CBF) is maintained (or increased) unless BP is markedly reduced.
• Increased cerebral blood volume leads to increased intracranial pressure (ICP), particularly in patients with reduced intracranial compliance (e.g., brain tumor).
• Intracranial hypertension may be minimized by slow administration and hyperventilation or hypocapnia.
Pulmonary: Vasculature dilates.
• Reductions in pulmonary artery pressure decrease perfusion to normally ventilated alveoli, thereby ![]() physiologic dead space =
physiologic dead space = ![]() V/Q mismatch and
V/Q mismatch and ![]() arterial oxygenation.
arterial oxygenation.
• Inhibits hypoxic pulmonary vasoconstriction: ![]() V/Q mismatch and
V/Q mismatch and ![]() arterial oxygenation.
arterial oxygenation.
Renal: Renin and catecholamines are released in response to ![]() BP
BP
Notable Drug Interactions
• Neuromuscular blockade: May indirectly delay the onset and prolong the duration of blockade by decreasing muscle blood flow secondary to arterial hypotension
Nitroglycerin: Mechanism of Action
• Same as sodium nitroprusside; relaxes vascular smooth muscle through NO pathway, with venous dilation predominating over arterial dilation
Clinical uses: Relieves myocardial ischemia, hypertension, and ventricular failure
• Commonly diluted to a concentration of 100 μg/mL
• Administered as a continuous IV infusion: 0.5 to 10 μg/kg/min
• Glass containers and special IV tubing are recommended because of adsorption of nitroglycerin to polyvinylchloride
• May also be administered by a sublingual (peak effect, 4 min) or transdermal (sustained release for 24 h) route
• Chronic use may result in tolerance: may be due to depletion of reactants necessary for NO formation, compensatory increase in vasoconstrictive substances, or volume expansion
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





