Fig. 45.1
CT scan of cerebrum after hypertonic saline bolus therapy
Fig. 45.2
MR imaging, T2-weighted, 14 days after correction of hyponatremia without signs of osmotic demyelination
Principles of Management
ABCD Followed by Diagnosis
The first step in the approach to the patient with an altered level of consciousness is to ensure a patent airway, adequate breathing and circulation together with a brief evaluation of the patient’s neurological disability and measurement of blood sugar (ABCD approach). This is crucial to treat/rule out hypoxia, hypercapnia, hypotension and/or hypoglycemia as causes behind the altered consciousness level and to reduce secondary brain damage [1]. Next, swift determination of electrolytes must be prioritized in the patient with severe cerebral symptoms together with the diagnosis/treatment of other possible causes of the cerebral symptoms (e.g. meningitis). The diagnosis of hypotonic hyponatremia is based on a reduced P[Na] without hyperglycemia. In the presence of hyperglycemia, the measured P[Na] should be corrected/increased by 0.4 mmol/l per 1 mmol increase in P-[glucose] (or a correction of 2.4 meq/l per 100 mg/dl increase in P-[glucose]) [2]. Hyponatremia together with severe symptoms indicates brain edema/increased intracranial pressure (ICP). That is, ongoing brain damage and a substantial risk of herniation. Therefore acute treatment should normally not be delayed by performance of a CTC. On the other hand, if hyponatremia is present without severe symptoms, a more hesitant approach is appropriate.
Bolus Therapy
In hyponatremia with severe symptoms, immediate ICP reduction is best induced with one or more boluses of 2 ml/kg 3 % (0.5 mmol/l) NaCl (e.g. 100 ml in a patient weighing 50 kg (110 lbs) (or a corresponding amount of more hypertonic NaCl) given iv/intraosseously (Fig. 45.3) [3–5]. One 2 ml/kg 3 % NaCl bolus causes an increase in P[Na] of approximately 2 mmol/l and an immediate reduction in ICP. The bolus can be repeated at 5-min intervals. Cerebral symptoms/ICP decreases sufficiently when P[Na] increases 4–6 mmol/l, hence no more than three boluses should be given [3, 6]. Crucially, 0.9 % NaCl should not be used to acutely increase P[Na] in patients with severe symptoms. The resulting P[Na] is unpredictable, and the hyponatremia may worsen in the patient with syndrome of inappropriate antidiuretic hormone (SIADH) (see section “Mechanisms Behind the Hyponatremia/Lasting Correction”) [6, 7].
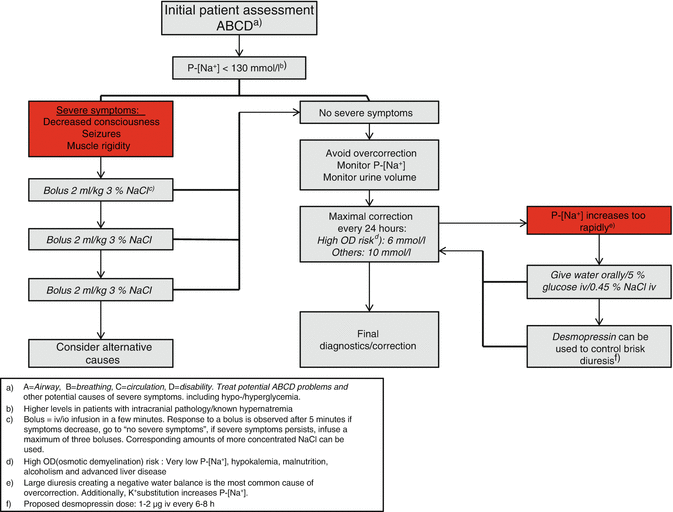

Fig. 45.3
Hyponatremia treatment algorithm. P-[Na + ] plasma sodium concentration, OD osmotic demyelination
Avoidance of Overcorrection
A rapid increase in P[Na] can result in osmotic brain damage and death, OD. In relation to hyponatremia, the course is bi-phasic: First, a reduction in cerebral symptoms followed by a gradual neurological worsening. OD is linked to the cerebral adaption to hyponatremia over time. Cerebral symptoms wane parallel with a reduced cerebral content of potassium, organic osmolytes and water. However, OD is also seen when a rapidly developed hyponatremia (Fig. 45.4) is swiftly corrected [8, 9] and in rapidly developed hypernatremia [3, 6]. Therefore, P[Na] should be corrected slowly in all patients. The increase in P[Na] should be of no more than 10 mmol/l in the first 24 h and less than 8 mmol/l every 24 h thereafter [3, 4]. In patients with additional risk factors of OD (very low P[Na], hypokalemia, malnutrition, alcoholism, and advanced liver disease), the increase should be maximally 6 mmol/l in every 24 h [3–5].
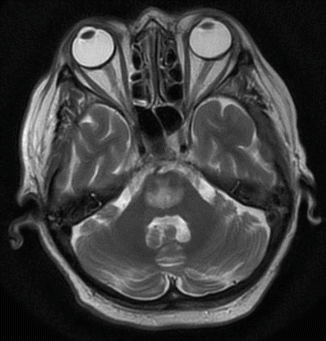

Fig. 45.4
MR imaging, T2-weighted, demonstrating osmotic demyelination in a 63-year-old woman after rapid correction of hospital-acquired hyponatremia (developed within 48 h)
Controlling the correction of hyponatremia can be a challenging task and needs a high level of observation. This is illustrated by the real-life case at the beginning of this chapter. First, it is crucial to state a maximal increase in P[Na]. Secondly, frequent measurements of P[Na] and urine volume are needed. Finally, practical measures should be instituted if P[Na] rises too quickly.
Understanding what determines P[Na] in the individual patient lies at the root of safe correction. Edelman demonstrated that P[Na] is determined by exchangeable sodium (eNa+), exchangeable potassium (eK+) and total body water (TBW) [10]. The relation is simplified in Eq. 45.1 [7]:
![$$ P\left[ N{a}^{+}\right]=\frac{eN{a}^{+}+ e{K}^{+}}{TBW} $$](/wp-content/uploads/2017/07/A329322_1_En_45_Chapter_Equ1.gif)
![$$ P\left[ N{a}^{+}\right]=\frac{eN{a}^{+}+ e{K}^{+}}{TBW} $$](/wp-content/uploads/2017/07/A329322_1_En_45_Chapter_Equ1.gif)
(45.1)
The physiological fundament for Eq. 45.1 is that sodium is the principal extracellular osmolyte, potassium is the principal intracellular osmolyte, and they do not freely cross the cell membrane in contrast to water. According to Eq. 45.1, hyponatremia develops when the proportion between cations (eNa+ + eK+) and water decreases.
Equation 45.1 is not readily useful at the bedside. However, it was recently demonstrated that it is valid in the individual and that the changes in P[Na] (changes from P[Na]1 to P[Na]2) are determined by changes in the external cation balances (Δ(Na+ + K+)) and water balances (ΔTBW) according to Eq. 45.2 [7, 11]:
![$$ \mathrm{P}-{\left[{\mathrm{Na}}^{+}\right]}_2=\frac{\mathrm{P}-{\left[{\mathrm{Na}}^{+}\right]}_1\times TBW+\varDelta \left( N{a}^{+}+{K}^{+}\right)}{TBW+\varDelta TBW} $$](/wp-content/uploads/2017/07/A329322_1_En_45_Chapter_Equ2.gif)
![$$ \mathrm{P}-{\left[{\mathrm{Na}}^{+}\right]}_2=\frac{\mathrm{P}-{\left[{\mathrm{Na}}^{+}\right]}_1\times TBW+\varDelta \left( N{a}^{+}+{K}^{+}\right)}{TBW+\varDelta TBW} $$](/wp-content/uploads/2017/07/A329322_1_En_45_Chapter_Equ2.gif)
(45.2)
In other words, P[Na] is influenced by the water balance and equally by the sodium and potassium balances. The primary cause of overcorrection in the hyponatremic patient (and in the case related above) is the large volume of diluted urine (diuresis) [12, 13]. Hypotonic fluids like water given orally or 5 % glucose iv can be used to counteract the effect of the diuresis. Besides monitoring P[Na], calculation of EFWC based on the urine cation concentration (U[Na] + U[K]) is useful because it quantifies the kidney’s impact on P[Na] [7]:
![$$ EFWC= uri{n}_{volume} x\left(1-\frac{\mathrm{U}-\left[ N{a}^{+}\right]+\mathrm{U}-\left[{K}^{+}\right]}{\mathrm{P}-\left[ N{a}^{+}\right]}\right) $$](/wp-content/uploads/2017/07/A329322_1_En_45_Chapter_Equ3.gif)
![$$ EFWC= uri{n}_{volume} x\left(1-\frac{\mathrm{U}-\left[ N{a}^{+}\right]+\mathrm{U}-\left[{K}^{+}\right]}{\mathrm{P}-\left[ N{a}^{+}\right]}\right) $$](/wp-content/uploads/2017/07/A329322_1_En_45_Chapter_Equ3.gif)
(45.3)
If electrolyte free water is excreted ((U[Na] + U[K]) < P[Na]), then the kidney counteracts the hyponatremia and will restore normonatremia. In this situation, a rapid increase in P[Na] may result. If the rise in P[Na] due to a large increase in urine volume (e.g. 300 ml/h)/high EFWC cannot be controlled, the V2-receptor agonist desmopressin is effective in reducing EFWC (e.g. 1–2 μg desmopressin iv every 6–8 h). The administration of desmopressin can also be necessary if P[Na] has increased too much, and a re-lowering to the desired level is needed [3–5].
However, it is important that the individual patient represents a dynamic system and urine volume can change dramatically in hours, leading to a rapid increase in P[Na] and making an exact prediction of P[Na] based on formulas uncertain [13, 14]. The safest way is close monitoring of P[Na] and urine output, especially if urine volumes are large, and adjustment of fluid treatment according to P[Na], EFWC and Eq. 45.2.
One special situation is the hyponatremic patient in need of acute dialysis. Here, measures must be taken to avoid rapid overcorrection, e.g. reducing the blood flow or diluting the fluids given [6].
Mechanisms Behind the Hyponatremia/Lasting Correction
When the initial therapy has stabilized the patient and measures to avoid overcorrection have been undertaken, mechanisms behind the hyponatremia must be identified. This can be challenging because (1) multiple combined causes are common [15, 16], (2) the initial mechanisms causing hyponatremia can be evanescent, and (3) hypovolemic and normovolemic hyponatremia (SIADH) can seldom be separated clinically but can be deduced from the response to treatment with 0.9 % NaCl [17]. Therefore, the diagnosis goes hand-in-hand with the treatment response and the causes of the hyponatremia may overlap.
To support the diagnosis, the patient’s history is important: current disease, exploration of thirst feeling, known comorbidities (e.g. heart failure, liver failure, and renal impairment), medications (Table 45.1) and a meticulous history of fluid intake (quantity/quality) and output (diuresis, gastrointestinal loss). The vital signs are determined together with the stigmata of chronic illness. A urine sample should be obtained as soon as possible – preferably before therapy is initiated – and analyzed for U[Na], U[K], osmolality to calculate EFWC and hence the kidneys’ contribution to the hyponatremia.
Table 45.1
Commonly prescribed drugs associated with hyponatremia
Groups | Drugs |
|---|---|
Diuretics | Thiazides Indapamide |
Antidepressant agents | Selective serotonin reuptake inhibitors |
Tricyclic antidepressants (mirtazapine) | |
Selective norepinephrine reuptake inhibitors Monoamine oxidase inhibitors | |
Antipsychotic agents | Phenothiazines, Butyrophenones |
Anti-seizure drugs | Carbamazapine, oxcarbazepine, valproate |
Lamotrigine Clofibrate | |
Antineoplastic agents | Alkylating agents (e.g. cyclophosphamide, ifosfamide) Platinium compounds (e.g. cisplatin) Vinca alkaloids (e.g. vincristine) Methotrexate |
V2-receptor agonist | Desmopressin, vasopressin, oxytocin |
Miscellaneous | Nonsteroidal anti-inflammatory drugs Opiates Voriconazole 3,4-methylenedioxy-methamphetamine (ecstacy) |
Hypotonic fluids |
Hyponatremia with Reduced ECV/Sodium
Loss of sodium (gastrointestinal, renal, blood, wounds, etc.) and thereby a reduction in extracellular volume (ECV) (sodium is the principal osmolyte in the ECV) results in hypovolemia and reduced perfusion with non-osmotic stimulation of antidiuretic hormone (ADH) secretion. ADH reduces renal water excretion (reduces EFWC). Ingestion/infusion of hypotonic fluids (e.g. water, 5 % glucose, KNaGlucose) in this situation can result in hyponatremia. With hypoperfusion, the renin-angiotensin-aldosterone system (RAAS) and the sympathetic nervous system are activated [18]. This results in reduced urine sodium excretion. However, a low U[Na] is not always present in patients on diuretic therapy or those with adrenal deficiency and/or metabolic alkalosis from vomiting. When the sodium/ECV deficit is restored (e.g. with 0.9 % NaCl/ringer-lactate), the ADH stimulus is abolished, and a high EFWC can result in a rapid P[Na] increase, with impending overcorrection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







