163 Hypoglycemia
• Every patient with an acute neurologic abnormality must be evaluated for hypoglycemia by rapid bedside measurement of the blood glucose concentration.
• The most common cause of hypoglycemia is exogenous insulin or sulfonylurea use as a treatment of diabetes, although hypoglycemia can be the initial feature of a large array of serious illnesses, including sepsis, liver disease, renal disease, and cancer.
• A blood glucose concentration lower than 60 mg/dL in a symptomatic patient should be corrected immediately with the administration of glucose, either orally or intravenously, or with intramuscular glucagon.
• Patients treated for hypoglycemia in the emergency department should be discharged only if a readily identifiable cause of the hypoglycemia was identified and if the risk for recurrence is minimal.
Epidemiology
Patients who use insulin or oral hypoglycemic medications are at the greatest risk for hypoglycemia. These patients experience mild, self-treated hypoglycemic episodes about twice per week.1 Severe hypoglycemia, which requires the assistance of another person to regain euglycemia, is experienced at least once per year by 27% of patients treated with intensive insulin regimens.2 Hypoglycemia is the cause of death in approximately 3% of people with insulin-dependent diabetes.3 Patients taking oral hypoglycemic agents also commonly experience hypoglycemia. Although these episodes are generally associated with milder symptoms, they occur in more than 30% of patients each year.4 Sulfonylureas are the most widely used oral hypoglycemic agents. In 2004, 4148 sulfonylurea overdoses were reported to American poison control centers; of these, 36% occurred in children younger than 6 years, 21% required treatment of hypoglycemia, 2.5% were life-threatening, and 0.22% were fatal.5 The incidence of hypoglycemia is expected to rise as tight glycemic control continues to be emphasized for the 17 million Americans with diabetes.
Normal Glycemic Control
Counterregulatory Hormones
Glycogenolysis increases blood glucose within minutes and can maintain euglycemia in a well-nourished person for 24 hours. Gluconeogenesis requires several hours to raise blood glucose levels and is the principal mechanism responsible for maintaining euglycemia if fasting is extended beyond 24 hours. Secretion of cortisol from the adrenal cortex and growth hormone from the anterior pituitary gland is a delayed response to blood glucose falling below 60 to 65 mg/dL; these hormones are not involved in the correction of acute hypoglycemia but act to maintain euglycemia over a period of days to weeks.6,7
Patients with type 1 and advanced (insulin-dependent) type 2 diabetes may have an impaired counterregulatory reaction. In these patients, the glucagon response is often nonexistent and the epinephrine response is greatly attenuated.8 This impaired counterregulatory response predisposes patients to severe hypoglycemia by blunting the glycemic response to falling blood glucose levels and thereby leaving the patient with no early warning symptoms of hypoglycemia. Furthermore, even one episode of hypoglycemia blunts the epinephrine response to future hypoglycemia and can result in hypoglycemia-associated autonomic failure.9 In this manner a vicious cycle of recurrent hypoglycemia can develop. Avoidance of hypoglycemia for several weeks improves hypoglycemia awareness and restores the epinephrine component of the counterregulatory response.8
Causes of Hypoglycemia
Hypoglycemia occurs in patients with a relative excess of insulin in comparison with the hormones of the counterregulatory response. Such excess can occur through the administration of exogenous insulin, an increase in endogenous insulin, or inhibition of the counterregulatory response. This section highlights the most important causes of hypoglycemia, the mechanisms by which they act, and the context in which they are likely to be observed in the emergency department (ED) (Table 163.1).
Table 163.1 Causes of Hypoglycemia
| CAUSE (EXAMPLES) | COMMENT |
|---|---|
| Exogenous insulin (treatment of diabetes or hyperkalemia, factitious disorder, Munchausen by proxy) | Hypoglycemia caused by excessive insulin administration. Most common cause of hypoglycemia |
| Oral hypoglycemic agents (sulfonylureas, meglitinides) | Induce secretion of insulin from pancreatic beta cells |
| Alcohol (ethanol) | Inhibition of hepatic gluconeogenesis. Hypoglycemia usually requires concomitant fasting |
| Sepsis | Inhibition of hepatic gluconeogenesis and increased peripheral glucose utilization |
| Liver disease (hepatitis from infections or toxins, cirrhosis, Reye syndrome, HELLP syndrome, hepatoma, metastatic tumors) | Inhibition of hepatic gluconeogenesis and glycogenolysis |
| Renal disease | Decreased clearance of insulin and reduced mobilization of gluconeogenic precursors |
| Congestive heart failure | Hepatic congestion causes inhibition of gluconeogenesis and glycogenolysis |
| Starvation (prolonged fasting, anorexia nervosa, pyloric stenosis, pediatric gastroenteritis) | Depletion of glycogen stores and gluconeogenic precursors |
| Hormone deficiency (cortisol, growth hormone, epinephrine, glucagon, hypopituitarism) | Failure of the counterregulatory mechanism of glucose metabolism. The hormone deficiency may be either congenital or acquired |
| Medications not used for the treatment of diabetes mellitus (ACE inhibitors, acetaminophen, acetazolamide, aluminum hydroxide, beta-blockers, benzodiazepines, Bordetella-pertussis vaccine, chloroquine, chlorpromazine, cimetidine, ciprofloxacin, colchicine, diphenhydramine, disopyramide, doxepin, ecstasy, EDTA, etomidate, ethionamide, fluoxetine, furosemide, haloperidol, imipramine, indomethacin, isoniazid, lidocaine, lithium, maprotiline, mefloquine, monoamine oxidase inhibitors, nefazodone, orphenadrine, pentamidine, phenytoin, propoxyphene, quinine, quinidine, ranitidine, ritodrine, selegiline, terbutaline, tetracyclines, trimethoprim-sulfamethoxazole, warfarin) | Induce hypoglycemia rarely and unpredictably, usually in otherwise healthy individuals |
| Insulinoma | Excessive, unregulated endogenous insulin secretion from a tumor of pancreatic beta-cell origin |
| Nesidioblastosis | Excessive insulin secretion by hypertrophic pancreatic beta cells |
| Non–islet cell tumors (sarcoma, carcinoid, melanoma, leukemia, hepatoma, teratoma, colon, breast, prostate, stomach, mesothelioma) | Various mechanisms, including secretion of insulin-like growth factors, increased metabolic demand, production of insulin autoantibodies |
| Post–gastric surgery status (gastric bypass, gastrectomy, pyloroplasty) | Rapid dumping of glucose into the small intestine causes an exaggerated insulin response; nesidioblastosis may have a role |
| Inborn errors of metabolism (errors in glycogen synthesis, glycogenolysis, gluconeogenesis, mitochondrial beta oxidation, amino acid metabolism) | Congenital defect prevents normal metabolism from maintaining euglycemia |
| Idiopathic ketotic hypoglycemia | Fasting intolerance, possibly caused by deficiency in alanine as a gluconeogenic precursor |
| Autoimmune | Antibodies against insulin or the insulin receptor augment the effects of insulin |
| Akee fruit | Unripe akee, a fruit found in Jamaica, contains toxins that inhibit hepatic gluconeogenesis |
| Vacor rat poison | Damages pancreatic beta cells, which initially causes release of insulin and hypoglycemia but eventually results in impaired insulin secretion and diabetes mellitus. Banned in the United States |
| Transient neonatal hypoglycemia (prematurity, intrauterine growth retardation, severe infant distress syndrome, perinatal asphyxia, maternal hyperglycemia, erythroblastosis fetalis, beta-agonist tocolytic agents) | Occurs in the immediate newborn period. Rarely seen in the ED |
| Persistent neonatal hypoglycemia (mutation in the sulfonylurea receptor gene, glutamate dehydrogenase gene, glucokinase gene) | Occurs in the immediate newborn period. Rarely seen in the ED |
ACE, Angiotensin-converting enzyme; ED, emergency department; EDTA, ethylenediaminetetraacetic acid; HELLP, hemolysis elevated liver enzymes, and low platelet count.
Exogenous Insulin and Oral Hypoglycemic Agents
Administration of insulin or an oral hypoglycemic medication is the most common cause of hypoglycemia. In a patient with diabetes who is being treated with an established regimen of insulin or an oral hypoglycemic medication, hypoglycemia can develop for a number of reasons (Table 163.2).
Table 163.2 Potential Causes of Hypoglycemia in a Diabetic Patient with an Established Regimen of Insulin or an Oral Hypoglycemic Agent
| MECHANISM | EXAMPLES |
|---|---|
| Decreased glucose availability | |
| Increased glucose use | |
| Increased dose of drug | |
| Increased availability of drug |
The time to peak effect and duration of action of insulin preparations and oral hypoglycemic medications dictate management and disposition (Table 163.3). Patients often cannot reliably recall which type of insulin they use; a helpful characteristic is that glargine and all rapid- and short-acting insulins are clear liquids whereas neutral protamine Hagedorn (NPH) and Ultralente appear cloudy.
Table 163.3 Features of Insulin Preparations and Oral Antidiabetic Agents Relevant to the Emergency Department Management of Hypoglycemia
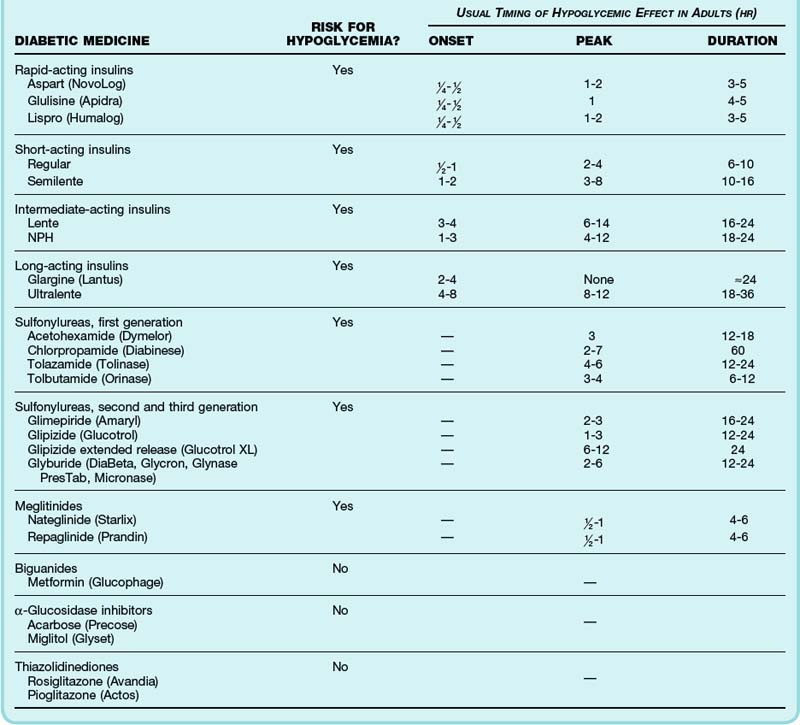
Additional Causes of Hypoglycemia
Alcohol ingestion (ethanol), the second most common cause of hypoglycemia in the ED, inhibits the counterregulatory response by suppressing hepatic gluconeogenesis. It has minimal effects on glycogenolysis. Therefore, alcohol typically requires concomitant fasting to deplete glycogen stores before hypoglycemia ensues. The classic example of alcohol-induced hypoglycemia is a malnourished alcoholic who undertakes a prolonged binge. However, fasting for only 6 hours before significant alcohol consumption in an otherwise healthy person can cause hypoglycemia. Although hypoglycemia is rare (less than 1%) in intoxicated patients in the ED,12,13 the hypoglycemic episodes seen in EDs in lower socioeconomic urban areas involve alcohol 50% of the time.14
Starvation, as in the case of anorexia nervosa, depletes glycogen stores and gluconeogenic precursors and can eventually lead to hypoglycemia. Hypoglycemia as a complication of anorexia nervosa is a late finding and implies a very grave prognosis.15
Insulinomas are tumors of pancreatic beta-cell origin that secrete insulin without the normal feedback mechanisms, thus producing unexplained hyperinsulinemia and hypoglycemia in otherwise healthy people. Insulinomas are rare, with an incidence of 4 per 1 million per year.16 Early diagnosis is important because these tumors are curable with surgery before they lead to potentially fatal hypoglycemia. Nesidioblastosis is characterized by hypertrophied (nonneoplastic) beta-cell tissue that oversecretes insulin and can also be manifested as unexplained hypoglycemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






