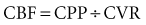88 Hypertensive Crisis
Emergency and Urgency
Hypertension is a common problem, and its incidence may be increasing in adults.1 Population data also suggest hypertension is increasing globally; 972 million individuals worldwide now have hypertension,1 and 30% of hypertensive individuals are unaware of their diagnosis.2 Of the 59% of hypertensive individuals being treated for hypertension, only 34% have a blood pressure less than 140/90 mm Hg.2 The exact risk of hypertensive crisis is not clear, but most authors estimate the risk to be less than 1%; it may be increasing.3,4
 Pathophysiology of Hypertensive Crisis
Pathophysiology of Hypertensive Crisis
The precise pathophysiology of hypertensive crisis is unknown. An abrupt increase in blood pressure is one of the initiating events in the transition from simple hypertension or normotension to hypertensive crisis. The product of cardiac output and peripheral vascular resistance determines blood pressure. The initial blood pressure increase is likely secondary to an increase in vascular resistance. Considerable evidence suggests that mechanical stress in the arteriolar wall leads to disruption of endothelial integrity.5 With disruption of vascular integrity, diffuse microvascular lesions develop.6,7 Fibrinoid necrosis of the arterioles is seen in vulnerable organs and is considered the histologic hallmark of hypertensive crisis.6,7 It is unclear whether hypertension alone causes the development to hypertensive crisis or whether other factors are necessary. For example, increases in peripheral vascular resistance result in part from activation of the renin-angiotensin-aldosterone system. Evidence suggests angiotensin II may directly injure the vascular wall by activation of genes for proinflammatory cytokines (interleukin 6) and also of nuclear factor κB.8,9 Other vascular-toxic influences may contribute to increased peripheral vascular resistance, including hyperviscosity, immunologic factors, and other hormones including catecholamines, vasopressin, and endothelin.10–12 The end result of these changes is a significant increase in peripheral vascular resistance, with ischemia of heart, brain, and kidneys.
 Diagnosis of Hypertensive Emergencies
Diagnosis of Hypertensive Emergencies
Medical History, Physical Examination, and Laboratory Evaluation
Hypertension from any cause may enter an “emergent” phase. Although hypertensive emergency usually occurs in individuals with a history of essential hypertension, it is also is seen in individuals with secondary hypertension and in individuals with no hypertensive history, as in preeclampsia, pheochromocytoma, drug withdrawal, and acute glomerulonephritis. A medication history, including over-the-counter medications and illegal drug use, should be ascertained from every patient. Malignant hypertension is a unique clinical/pathologic syndrome that is associated with hypertensive crisis. Increases in blood pressure and target-organ damage are caused by changes in the vasculature characterized by fibrinoid necrosis and a proliferative endarteritis. Risk factors associated with the development of malignant hypertension include age between 30 and 50 years,13 male gender,5 African American background,14 and smoking (increases the risk by 2.5- to 5-fold).15
Patients with hypertensive crisis present with a variety of symptoms. The most common is headache. It is either sudden in onset or represents a change from a usual headache pattern and is often worst in the morning. The location is generally occipital or anterior, with a steady quality. Other symptoms include visual complaints (scotoma, diplopia, hemianopsia, blindness), neurologic symptoms (focal deficits, stroke, transient ischemic attacks, confusion, somnolence), ischemic chest pain, renal symptoms (nocturia, polyuria, hematuria), back pain (aortic aneurysm), and gastrointestinal complaints (nausea, vomiting). Weight loss occurs as the high levels of circulating renin and angiotensin induce a diuresis.16 These patients often present with intravascular volume depletion, which has strong implications for treatment.
The blood pressure is measured in both arms and also with the patient lying and standing. In hypertensive emergency, diastolic blood pressures are usually above 120 mm Hg. Pathologic processes that cause stiffening of the vascular wall can prevent vessel compression by external compression with a blood pressure cuff. This results in an artificial increase (at times extreme) in the systolic and diastolic blood pressure, or “pseudohypertension.” Pseudohypertension can occur in atherosclerosis, Monckeberg’s medial calcification, and metastatic calcification, as experienced in end-stage renal disease. Clues to pseudohypertension include a markedly elevated blood pressure in an individual without evidence of end-organ damage. The diagnosis is suggested by a palpable radial artery after proximal compression (Osler’s maneuver).17
A dilated funduscopic examination should be performed on all individuals. Arteriolar thickening reflects chronic hypertension and is manifested by increased light reflex, vascular tortuosity, and arteriovenous nicking where the arterioles cross the venules. These funduscopic findings reflect chronic hypertension and have no prognostic significance with regard to hypertensive crisis. As hypertension increases in severity, there are additional findings caused by the breakdown of the blood-retina barrier, leading to retinal hemorrhage and leakage of lipids, causing hard exudates. Additional findings as the blood pressure continues to increase may include cotton-wool spots as a result of nerve ischemia and swelling of the optic nerve with papilledema.18
The initial laboratory evaluation should include a serum sodium, chloride, potassium, bicarbonate, creatinine and blood urea nitrogen, complete blood count (with a peripheral smear to identify schistocytes), prothrombin time, activated partial thromboplastin time, serum and urine toxicology screen, pregnancy test when appropriate, an electrocardiogram, and a urinalysis. Evidence of intravascular hemolysis is common and may make it difficult to differentiate hypertensive crisis from primary vasculitis with secondary hypertension.19,20 The renin-angiotensin-aldosterone axis is markedly activated, as evidenced by hypokalemia and metabolic alkalosis.3,21 The blood urea nitrogen and creatinine are often elevated. The urinalysis may show small amounts of proteinuria as well as hematuria with occasional erythrocyte casts.5 Marked increases in proteinuria suggest a primary glomerular process such as glomerulonephritis as the etiology of the elevated blood pressure.
If hypertensive encephalopathy is suspected, magnetic resonance imaging (MRI) should be performed. With hypertensive encephalopathy, edema may occur in the posterior regions of the cerebral hemispheres, particularly in the parieto-occipital regions, a finding called posterior leukoencephalopathy on MRI. However, brainstem involvement on MRI has also been reported.22,23 It is important to consider and eliminate other conditions with a similar clinical presentation (Box 88-1). Several important diagnostic considerations help exclude other causes of altered mental status: (1) symptoms of generalized brain dysfunction tend to develop over time (12-24 hours) with hypertensive encephalopathy, as compared to acutely with ischemic stroke or cerebral hemorrhage; (2) focal neurologic findings are unusual with hypertensive encephalopathy unless there is an associated bleed; (3) papilledema is almost always noted with hypertensive encephalopathy and if absent should raise suspicion of another etiology; (4) in comparison to an acute CNS bleed, mental status with hypertensive encephalopathy improves within 24 to 48 hours of treatment.
 Treatment of Hypertensive Emergency
Treatment of Hypertensive Emergency
In most but not all settings, blood pressure can be reduced acutely by 20% to 25% within minutes to hours.3 After the patient is stabilized at this pressure, the blood pressure may be further decreased to 160/100-110 mm Hg over the next 2 to 6 hours.3 If the patient is clinically stable, the blood pressure may then be decreased toward a normal blood pressure over the next 24 to 48 hours.3 With these decreases in blood pressure, CNS blood flow autoregulation is usually maintained. Clinical settings where additional considerations and alternative approaches to reducing blood pressure should be considered include (1) ischemic stroke where immediate reduction of blood pressure is usually not indicated except when the blood pressure is over 220/120 or the patient requires thrombolytic therapy, (2)acute aortic dissection where a rapid blood pressure reduction in 15 to 30 minutes to a systolic blood pressure under 100 mm Hg is clinically warranted if the patient tolerates, and (3) in previously normotensive subjects with abrupt increases in BP.
From 40% to 50% of hypertensive crises arise in patients with preexisting hypertension without identifiable secondary causes.24,25 Essential hypertension is the underlying disorder in the majority of African American individuals.26–28 In contrast, from 50% to 60% of white patients with malignant hypertension have an identifiable cause (Box 88-2). Renovascular hypertension secondary to either fibromuscular dysplasia or atherosclerosis is not uncommon. Up to 20% of cases of malignant hypertension occur in patients with underlying chronic glomerulonephritis. Other renal causes include reflex nephropathy (particularly in children) and analgesic nephropathy.3
 Specific Treatment Recommendations for Hypertensive Crisis Based on Etiology
Specific Treatment Recommendations for Hypertensive Crisis Based on Etiology
General Comment on Medication Used to Treat Hypertensive Crisis
The classes of parenteral antihypertensive agents available to treat hypertensive crisis include direct vasodilators (sodium nitroprusside, nitroglycerin), α- and β-adrenergic blockers (labetalol), α-adrenergic blockade (phentolamine), angiotensin-converting enzyme (ACE) inhibitors (enalaprilat), calcium channel blockers (nicardipine), and dopamine agonists (fenoldopam). Some of the advantages and disadvantages of these medications are detailed in Table 88-1. There is no consensus on the most effective antihypertensive medications in the setting of a CNS insult and no large randomized trials demonstrating the superiority of a given agent. Rather, the choice of antihypertensive therapy should be individualized to the patient and clinical setting. However, most authors now caution the use of nitroprusside in the setting of increase in intracranial pressure. Vasodilators increase blood volume and therefore have the potential to increase the intracranial pressure (ICP). Animal and human studies in the setting of a normal ICP show no effect of nitroprusside on ICP.19–21 However, in studies on animals and humans with preexisting increased ICP, nitroprusside increased the ICP, likely reflecting vasodilatation on the background of decreased cranial compliance.29–33 When sodium nitroprusside is contraindicated, other treatment options include labetalol and nicardipine. Fenoldopam, which is an agonist of the vasodilator dopamine-1 receptor, shares with nitroprusside a rapid onset and short duration of action. In addition, fenoldopam, in contrast to nitroprusside, increases renal blood flow, induces natruresis, and produces no toxic metabolites.34–38
TABLE 88-1 Treatment of Hypertensive Crisis: Intravenous Medication
| Drug Name and Mechanism of Action | Indications/Advantages/Dose | Disadvantages/Adverse Effects/Metabolism Cautions |
|---|---|---|
| Sodium nitroprusside: Nitric oxide compound; vasodilation of arteriolar and venous smooth muscle Increases cardiac output by decreasing afterload | Useful in most hypertensive crisis Onset of action immediate, duration of action 1-2 min Dose: 0.25 µg/kg/min Maximum dose: 8-10 µg/kg/min | Contraindicated in high-output cardiac failure, congenital optic atrophy. Anemia and liver disease at risk of cyanide toxicity: acidosis, tachycardia, change in mental status, almond smell on breath. Renal disease at risk of thiocyanate toxicity: psychosis, hyperreflexia, seizure, tinnitus. Cautious use with increased intracranial pressure. Do not use maximum dose for more than 10 minutes. Crosses the placenta. |
| Nitroglycerin: Directly interacts with nitrate receptors on vascular smooth muscle Primarily dilates venous bed Decreases preload | Use with symptoms of cardiac ischemia, perioperative hypertension in cardiac surgery Initial dose: 5 µg/min Maximum dose: 100 µg/min | Contraindicated in angle-closure glaucoma, increased intracranial pressure. Blood pressure decreased secondary to decreased preload, cardiac output—avoid when cerebral or renal perfusion compromised. Caution with right ventricular infarct. |
| Labetalol: β-Adrenergic blockade and α-adrenergic blockade IV α:β-Blocking ratio is 1 : 7 | Onset of action 2-5 min Duration 3-6 hours Bolus 20 mg, then 20-80 mg every 10 min for maximum dose 300 mg Infuse at 0.5-2 mg/min | Avoid in bronchospasm, bradycardia, congestive heart failure, greater than first-degree heart block, second/third trimester pregnancy. Use caution with hepatic dysfunction, inhalational anesthetics (myocardial depression). Enters breast milk. |
| Esmolol: Cardioselective β1-adrenergic blocking agent | Use with aortic dissection Use during intubation, intraoperative, and postoperative hypertension Onset 60 seconds, duration 10-20 min 200-500 µg/kg/min for 4 min, then infuse 50-300 µg/kg/min | See labetalol. Not dependent on renal or hepatic function for metabolism (metabolized by hydrolysis in RBC). |
| Fenoldopam: Postsynaptic dopamine-1 agonist; decreases peripheral vascular resistance; 10 times more potent than dopamine as vasodilator | May be advantageous in kidney disease, increases renal blood flow, increases sodium excretion, no toxic metabolites Initial dose: 0.1 µg/kg/min, with titration every 15 min No bolus | Contraindicated in glaucoma (may increase intraocular pressure) or allergy to sulfites; hypotension, especially with concurrent beta-blocker. Check serum potassium every 6 hours. Concurrent acetaminophen may significantly increase blood levels. Dose-related tachycardia. |
| Hydralazine: Primarily dilates arteriolar vasculature | Primarily used in pregnancy/eclampsia Dose: 10 mg every 20-130 min; maximum dose 20 mg Decreases blood pressure in 10-20 min Duration of action 2-4 h | Reflex tachycardia; give beta-blocker concurrently. May exacerbate angina. Half-life 3 hours, affects blood pressure for 100 hours. Depends on hepatic acetylation for inactivation. |
| Phentolamine: α-Adrenergic blockade | Used primarily to treat hypertension from excessive catecholamine excess (e.g., pheochromocytoma) Dose: 5-15 mg Onset of action 1-2 min, duration 3-10 min | β-blockade is generally added to control tachycardia or arrhythmias. As in all catecholamine excess states, beta-blockers should never be given first, as the loss of β-adrenergically mediated vasodilatation will leave α-adrenergically mediated vasoconstriction unopposed and result in increased pressure. |
| Nicardipine: Dihydropyridine calcium channel blocker; inhibits transmembrane influx of calcium ions into cardiac and smooth muscle | Onset of action 10-20 min, duration 1-4 h Initial dose: 5 mg/h to maximum of 15 mg/h | Avoid with congestive heart failure, cardiac ischemia. Adverse effects include tachycardia, flushing, HA. |
| Clevidipine: Short-acting dihydropyridine calcium channel hypertension99 | Initial dose: 1 mg/h; can be increased to 21 mg/h | Reduces blood pressure without affecting cardiac filling pressures or causing reflex tachycardia |
| Enalaprilat: Angiotensin-converting enzyme inhibitor | Onset of action 15-20 min, duration 12-24 h Dose: 1.25-5 mg every 6 h | Response not predictable, with high renin states may see acute hypotension. Hyperkalemia in setting of reduced glomerular filtration rate. Avoid in pregnancy. |
| Trimethaphan:Nondepolarizing ganglionic blocking agent; competes with acetylcholine for postsynaptic receptors | Used in aortic dissection Dose: 0.5-5 mg/min | Does not increase cardiac output. No inotropic cardiac effect. Disadvantages include parasympathetic blockade, resulting in paralytic ileus and bladder atony, and development of tachyphylaxis after 24-96 hours of use. |
Malignant Hypertension
Malignant hypertension is specific syndrome characterized by markedly elevated pressures in conjunction with hypertensive neuroretinopathy. Funduscopic examination often reveals flame-shaped hemorrhages, cotton-wool spots, or papilledema. Malignant hypertension is also associated with nephropathy, encephalopathy, microangiopathic hemolytic anemia, and cardiac ischemia. Untreated malignant hypertension is a rapidly fatal disorder, with a mortality of more than 90 % within 1 year, as reported in a classic series by Kincaid-Smith.6 In this series, deaths were due to renal failure (19%), congestive heart failure (13%), renal failure plus congestive heart failure (48%), stroke (20%), and myocardial infarction (1%).
Aggressive therapy to prevent progressive ischemic injury in malignant hypertension is critical. Although the autoregulatory range of CNS blood flow is reset upwards in chronic hypertension, the lower limit of the autoregulation remains approximately 25% below the resting mean blood pressure in patients with both normotension and chronic hypertension.39 When the arterial blood pressure falls below this lower limit, cerebral blood flow decreases progressively, and symptoms of low CNS flow including nausea, yawning, hyperventilation, clamminess, and syncope develop. To protect cerebral function, after initial reduction of blood pressure by 20% within the first hour, blood pressure is further reduced over the next 2 to 6 hours to the 160/110 range as long as the patients remains stable. Nitroprusside is one of the most useful intravenous agents for hypertensive emergency. Some patients are highly sensitive to treatment owing to coexisting hypovolemia; therefore, low-dose nitroprusside (0.3 µg/kg/min or less, with titration every 3-5 minutes) is used to reach goal blood pressure. A number of parenteral agents have been used as successful alternatives to nitroprusside, including labetalol, fenoldopam, and nicardipine. Premature discontinuation of parenteral therapy may cause rebound hypertension. Oral therapy is usually started after the pressure has been stabilized on parenteral therapy. Parenteral therapy is then slowly weaned.
Renal failure is common with malignant hypertension. For patients with worsening renal failure due to malignant hypertension, renal failure exacerbates the hypertension. Aggressive treatment can arrest and reverse renal damage. Since the arteriolopathy of malignant hypertension includes fixed anatomic lesions, initial lowering of blood pressure may worsen renal function. Dialysis may be required in patients with a presenting creatinine greater than 4.5 mg/dL.40 In the majority of patients, renal function begins to improve after 2 weeks of therapy. Of the patients who require dialysis, 50% will regain sufficient function to discontinue dialysis.41 Recovery of renal function is predicted when the combined length of both kidneys is 20.2 cm or more, but is felt to be unlikely when the length is 14.2 or less.42 The mean time to recovery is approximately 2 to 3 months, but recovery after up to 26 months has been reported.43 In patients with malignant hypertension secondary to glomerulonephritis, eventual deterioration to end-stage renal disease (ESRD) may occur despite blood pressure control.44 In contrast, renal function tends to remain well preserved in patients without underlying glomerulonephritis if blood pressure is well controlled. Nitroprusside has been one of the preferred agents to treat hypertension and renal failure. The metabolism of nitroprusside results in the production of cyanide, which is taken up by red blood cells and conjugated to thiocyanate in the liver. Cyanide toxicity occurs in patients with anemia or liver disease, whereas thiocyanate toxicity is seen in the setting of renal disease (see Table 88-1). Thiocyanate levels should be monitored and the duration of therapy kept to less than 72 hours whenever possible. Fenoldopam has no toxic metabolites and may protect renal function.34–38
Controversy exists as to the management of the relatively asymptomatic malignant hypertensive patient (i.e., with neuroretinopathy alone).45,46 Although oral medication under close observation has been used successfully,47 we prefer initial parenteral therapy. The progressive breakdown of CNS autoregulation in these patients enhances the sensitivity to ischemia, with abrupt decreases in blood pressure. Of the oral agents, calcium antagonists and minoxidil are effective and safe. ACE inhibitors may cause hyperkalemia in undialyzed patients with significant renal insufficiency.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree