152 Hydrocarbons
• Many hydrocarbons possess a characteristic odor similar to that of gasoline or lighter fluid. Solvents containing aldehyde or ketone groups smell sweet or fruity, and essential oils are characteristically pungent or aromatic. In acutely intoxicated patients, these odors are rarely missed.
• In assessing the patient with possible hydrocarbon exposure, important tasks are as follows:
• Many hydrocarbons are acutely cardiotoxic and have a propensity to induce tachyarrhythmias by sensitizing the myocardium to the arrhythmogenic effects of catecholamines.
• Gaseous or volatilized hydrocarbons are likely to cause toxicity through inhalation.
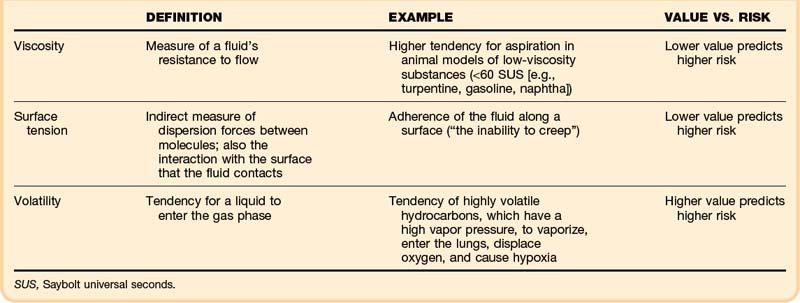
• Viscosity, surface tension, and volatility determine the aspiration potential and the risk of pulmonary toxicity.
Pathophysiology
Lipid-soluble solvents (aromatic, aliphatic, or halogenated hydrocarbons) are more likely than water-soluble hydrocarbons (alcohols, ketones, or esters) to cause acute central nervous system (CNS) effects. Clinicians are familiar with these effects from experience with inhaled anesthetic agents, which cause CNS sedation similar to that resulting from other hydrocarbons. The Meyer-Overton hypothesis suggests that inhaled anesthetics dissolve into some critical lipid compartment of the CNS and cause generalized inhibition of neuronal transmission. This mechanism is probably oversimplified, but it helps to explain partly the nonspecific inhibition of neuronal transmission that hydrocarbons produce in the CNS. Specific membrane interactions may also contribute to this process,1 and several receptor-mediated interactions are known to occur.
Specific physical properties of ingested hydrocarbons help to predict the risk of pulmonary aspiration (Table 152.1). In particular, viscosity, surface tension, and volatility determine aspiration potential and the contribution to pulmonary toxicity.2 Viscosity is a measure of a fluid’s resistance to flow, commonly described in units of Saybolt universal seconds (SUS). This property is not the same as the fluid’s density; in fact, these two properties correlate poorly.
Surface tension indirectly measures dispersion forces between molecules in a fluid, but it also characterizes the interaction with the surface that the fluid contacts. This property can be quantified on a modified Wilhelmy balance, which measures adherence of the fluid along a surface (“the inability to creep”). In theory, the lower the surface tension is, the higher is the aspiration risk.2 A lower surface tension value predicts a higher risk.
Mechanisms of Toxicity
Although organ-specific pathophysiology is often unique to individual agents, much of the toxicity of hydrocarbons results from their ability to dissolve fats or, similarly, to diffuse across hydrophobic barriers intended to protect anatomic structures (e.g., lipid bilayers, myelin). Hydrocarbon solvents cause irritation of skin and mucous membranes. Recurrent or prolonged contact results in “defatting” of skin, dissolving lipid components, and disrupting the normal architecture of the stratum corneum.3
Skin Effects
The skin is a common site of contact and a potential portal of entry for hydrocarbons. Skin is composed of both hydrophilic and hydrophobic elements. Agents that contain both hydrophobic and hydrophilic regions (glycol ethers, dimethylformamide, dimethylsulfoxide) are highly absorbed. Dermal absorption usually constitutes a small fraction of the hydrocarbon dose absorbed by other routes (e.g., inhalation), however. The absorbed dose depends on the surface area exposed, the duration of contact, and skin’s integrity (e.g., cut, abraded).5
Pulmonary Effects
Severe hydrocarbon pneumonitis also results from intravenous injection of a hydrocarbon. In animals, intravascular hydrocarbons injure the first capillary bed encountered. The clinical course after intravenous hydrocarbon exposure mirrors that of aspiration injury.5
Cardiac Effects
Endogenous or exogenous catecholamines (e.g., epinephrine) are proarrhythmic. Hydrocarbons enhance this potential and are said to sensitize the myocardium to the arrhythmogenic effects of catecholamines. Essentially every class of hydrocarbon compounds, including general anesthetic agents, can sensitize the heart. Some classes carry a high risk, however, and others sensitize the myocardium modestly, if at all. The ability of these substances to sensitize the heart constitutes an accepted system for grading halocarbon (e.g., Freon) toxicity. Unsaturated, aliphatic hydrocarbons (e.g., ethylene) and aliphatic ethers have been studied but do not appear to be sensitizers. Other unsaturated compounds (e.g., acetylene), are weak sensitizers. Aromatic hydrocarbons and, especially, halogenated hydrocarbons are often potent sensitizers.6
Sensitization appears to be mediated by slowed conduction velocity, possibly by chemical and functional changes in the membrane transport proteins at gap junctions. The major ventricular gap junction protein is composed of connexin 43. This protein is regulated by phosphorylation, such that the dephosphorylated state of the hexamers in the channel is associated with greater gap junction resistance. In the presence of epinephrine, halocarbons increase gap junction resistance in myocardial tissue and slow conduction velocity.7
Nervous System Effects
The mechanism by which hydrocarbons depress consciousness is unknown. Diffusion across the blood-brain barrier with neuronal membrane stabilization provides the foundation of the Meyer-Overton hypothesis. To date, no specific receptor wholly explains this generalized effect. In cases of pulmonary toxicity, hypoxemia may contribute to depressed consciousness.8
Chronic solvent abuse leads to irreversible CNS toxicity, best described in the setting of toluene abuse. Volitional abusers demonstrate loss of cerebral white matter, with a characteristic syndrome of cognitive and motor deficits. Autopsied brains of long-term toluene abusers show profound atrophy and mottling of the white matter, as though the lipid-based myelin had been dissolved away. Microscopic examination shows a consistent pattern of demyelination, with relative preservation of axons. These pathologic features correlate with the clinical syndrome of subcortical dementia.9 Mild cognitive deficits show improvement after 6 months of abstinence. In patients with advanced disease, regardless of the exposure history, full recovery is unlikely.10
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree