Sexual Pain Disorders
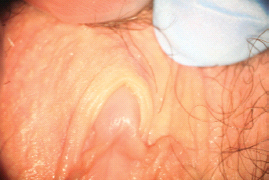
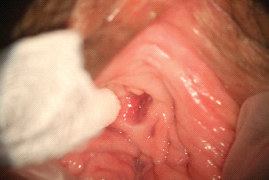
There are multiple proposed pathophysiological processes, both psychological and biological, involved in female sexual pain disorders [1–16]. These disorders involve central and peripheral genital/reproductive tissues, including specific central nervous system nuclei such as the medial preoptic area, nucleus paragigantocellularis, paraventricular nucleus, and specific peripheral structures such as the mons pubis, labia majora, labia minora, clitoris, prepuce, frenulum, labia minora, hymen, vulvar vestibule, minor vestibular glands, Skene’s glands, urethral meatus, and vagina (Figures 28.1–28.3).
Central [17–23] and peripheral genital tissues [24–31] utilize sex steroid hormones (androgens, estrogens, and progestins) for structure and function. Thus, it is possible that sexual pain disorders can occur in women with abnormalities in sex steroid hormones. This chapter reviews the measurement of sex steroid hormones, the physiology and pathophysiology of sex steroids, and the treatment of women with sexual dysfunction. When relevant, hormones and sexual pain disorders–provoked vestibulodynia (PVD) and atrophic vaginitis [32–41]–are examined.
Clinical Measurement of Sex Steroids
Currently, there is no consensus regarding sex steroid hormone blood tests for women’s sexual health concerns. Because there are several types of androgens, estrogens, and progesterone, multiple sex steroid hormones should be measured in women with all types of sexual dysfunction, including dyspareunia. In addition, other endocrine assessments should be pursued; pituitary function can be assessed by determination of prolactin, follicle-stimulating hormone (FSH), and lutenizing hormone (LH) levels, and thyroid function can be assessed by measuring thyroid-stimulating hormone levels [42, 43].
The following androgen values may be measured if considered appropriate in women with sexual dysfunctions: dehydroepiandrosterone sulphate (DHEA-S), androstenedione, total testosterone, and dihydrotestosterone. Assessment of DHEA-S and androstenedione values may provide insight as to the function of the adrenal gland zona reticularis. DHEA is usually measured in the sulfated form, DHEA-S, because the half-life is much longer, resulting in more stable levels. Both DHEA-S and androstenedione levels remain stable over the various phases of the menstrual cycle [44]. The glycoprotein, sex hormone binding globulin (SHBG)should be measured if the woman with dyspareunia has taken exogenous estrogen steroids, such as ethinyl estradiol (in oral contraceptive pills) [45–48] or estradiol (in menopausal hormone therapy) [49, 50], or has a history of hyperthyroidism [51], liver disease [52], anorexia nervosa [53], or other nonestradiol drug use (e.g., phentoin) [54].
These conditions are recognized causes of increased SHBG values. It is clinically important to record SHBG values in women with dyspareunia as this globulin binds to testosterone. Androgens circulate in the bloodstream, bound mostly to SHBG and to serum albumin. Only 1–2% of androgens are unbound, and thus biologically active to enter cells in structures such as the clitoris, vagina, and minor vestibular glands and activate the cytoplasmic androgen receptor. This hormone–receptor complex becomes a transcriptional agent that enters the nucleus and induces protein synthesis [27]. Critical proteins such as vascular endothelial growth factor, nerve growth factor, and nitric oxide synthase are androgen-dependent. Of note, SHBG also binds estradiol, while progesterone is bound by transcortin.
When recording androgen levels in women with sexual pain, several caveats should be mentioned. There are universal accuracy limitations to the measurement of total testosterone in both genders [55]. In women–who physiologically have approximately 10% of the testosterone values as compared to men–it is recognized that more accurate and reliable testosterone assays are needed. The U.S. Food and Drug Administration, however, haspermitted equilibrium dialysis free testosterone and bioavailable testosterone assays in clinical trials of testosterone treatment trials in women [56].
Figure 28.2 Normal anatomy of the perineal region.
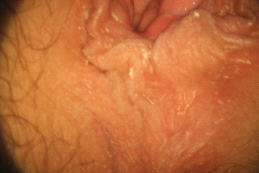
Several laboratories offer mass spectroscopy after column chromatography separation of the steroids. Mass spectroscopy is utilized to obtain a higher level of accuracy with total testosterone measurements [57]. This is relevant especially because equilibrium dialysis free testosterone and the bioavailable testosterone measurements depend on the accuracy of the total testosterone determination. In women with sexual pain, a calculated free testosterone value may also be determined [58–60], which takes into account total testosterone, SHBG, and albumin. A calculator for this value is available online at http://www.issam.ch/freetesto.htm. This method has high reliability and sensitivity to equilibrium dialysis-free testosterone values [58–60].
Unbound free testosterone values may also be estimated using the free androgen index, which is defined as the total testosterone concentration (in nmol/L; multiply testosterone units in ng/dL × 3.47 = nmol/L) divided by the concentration of SHBG (in nmol/L). Finally, the timing of the measurement may be of importance, especially in premenopausal women. Testosterone levels reach a peak during the early follicular phase, with small but less significant variation across the rest of the cycle [44]. Thus, blood may be drawn after day 8 of the cycle, and preferably before day 20.
Sex steroid hormones undergo metabolism by critical intracellular cytosolic enzymes (5-α-reductase) and undergo binding to critical cytosolic hormone receptors (androgen receptors) for physiologic genomic action. These cytoplasmic and nuclear physiologic processes are variable in individuals and determine individual tissue exposure, tissue sensitivity, and tissue responsiveness. For example, within individuals, there are variations in the activity of critical cytosolic enzymes such as 5-α-reductase. There are also variations in androgen receptor sequencing within individuals. For example, the number of repeat sequences of cytosine, adenine, and guanine nucleotides (CAG repeats) in the deoxyribonucleic acid molecule coding for the sex steroid hormone receptor may vary widely in individuals [61]. Thus, independent of the values of plasma testosterone values, the unique variations in critical enzymes and sex steroid hormone receptors result in individual differences in how testosterone is utilized and metabolized. So, the health care provider needs to be aware that measurement of plasma testosterone values are only the tests “currently clinically available.” In the future, there will be better, and more accurate, sensitive, and clinically meaningful measurements of androgen metabolism in women [62].
The blood test for dihydrotestosterone may be of interest if women with dyspareunia report side effects of acne [63] or alopecia [64] following treatment with testosterone for the management of androgen insufficiency syndrome associated with oral contraceptive pill use. If dihydrotestosterone is elevated, a 5α-reductase inhibitor may be considered to lower the level and reduce the symptoms.
Estrogen values such as estradiol and estrone may be assessed with the understanding that estradiol is the most biologically active form of estrogen. It should be noted that the blood levels of estradiol may not reflect exogenous estrogen administration in women on exogenous conjugated equine estrogens or exogenous ethinyl estradiol, since the latter estrogens are not bio-identical and therefore cannot be accurately assessed in serum. If a woman with sexual pain is perimenopausal, three determinations of estradiol, progesterone, LH, and FSH every 10 days during a month, will assess ovarian and ovulatory integrity.
The “normal range” for sex steroid blood test values in women with sexual pain has not been defined. Normative androgen data for women without sexual health problems have been published in a large study of over 1,400 women (aged 18–75) separated into age-related cohorts [65]. Normative androgen data have also been reported in a subset of premenopausal healthy women without sexual health concerns [66]. Both normative data sets correlate well with each other.
Concerning the relation of androgens to aging, a prospective longitudinal study of serum testosterone, DHEA-S, and SHBG levels through the menopause transition [67], as well as a study of androgen levels in women of all ages using mass spectrometry [68] both demonstrated a marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites with increasing age.
DHEA-S and total and unbound testosterone values also decrease with age in women [67, 69, 70]. Zumoff et al. [70] reported that the testosterone concentration of women aged 20–29 years was twice the value of women aged 40–49 years. Guay et al. [66] examined androgen values in women “without sexual dysfunction.” Androgen concentrations were highest in the women aged 20–29 years. The calculated free testosterone in these women was 0.6–0.8 ng/dL for women aged 20–29 years and 0.4–0.6 ng/dL for women between the ages of 30 and 49 years.
Androgens: Physiology and Pathophysiology
DHEA is an adrenal precursor sex steroid hormone that is converted to other androgens, such as δ androstenediol, δ4-and rostenedione, and testosterone via the enzymes 3β-and 17β-hydroxysteroid dehydrogenase, and ultimately to estradiol via aromatase or to dihydrotestosterone via 5α-reductase. Any positive effects of DHEA on sexual function must take into account all the actions of DHEA: DHEA alone, and as a precursor of androgens and estrogens. DHEA receptors have been found on endothelial cells, implying that DHEA is involved in the process of vascular smooth muscle relaxation [71]. The physiologic process of vascular smooth muscle relaxation is intimately involved with peripheral sexual arousal [27].
δ5-androstenediol acts on its own receptors on the vaginal mucosa and is involved in the mucin content of vaginal lubrication [72, 73]. It may be possible that low values of δ5-androstenediol are associated with sexual arousal and sexual pain disorders.
Testosterone has been linked to the central regulation of female sexual behavior. Animal studies reveal that neurons containing androgen receptors are widely distributed in the hypothalamus and telencephalon, which are thought to play a key role mediating the hormonal control of sexual behavior. These regions provide strong input to the medial preoptic and ventromedial nuclei, areas also associated with sexual behavior [74–77].
Androgens are critical in maintaining peripheral genital tissue structure and function [27]. Androstenedione and testosterone levels have been shown to be linked to vaginal physiologic function [72, 73]. Androgen receptors have been reported in the vagina and vulvar vestibule [78, 79]; these may play a critical role in vaginal and vulvar health. Multiple preclinical studies in ovariectomized animals (rabbits and rats) have been performed [27]. These investigations have shown that androgen treatment enhances vaginal tissue nitric oxide synthase expression and activity, facilitates vaginal smooth muscle relaxation, increases vaginal blood flow [72], enhances vaginal mucification [73], and maintains the health and integrity of the vaginal muscularis layer [27]. Androgens contribute to other sexual and nonsexual physiologic functions, such as bone and skeletal muscle metabolism, cognition, energy, and feelings of well-being [80].
In premenopausal women with regular menstrual cycles, there is a rise in testosterone and androstenedione in the late follicular phase of the menstrual cycle and in the luteal phase. In women, approximately 50% of testosterone synthesis occurs directly in the ovaries and in the adrenal glands, and the remaining 50% occurs from testosterone precursors such as androstenedione and DHEA in the peripheral tissues.
There is a slow and progressive decline in serum testosterone levels as women age [66–70], which is in sharp contrast to estrogen and progesterone levels that fall abruptly with menopause. In the late reproductive years, the midcycle rise in free testosterone, a hallmark of the menstrual cycle in young ovulating women, begins to diminish. The level of adrenal precursors that serve as a prehormone for approximately half of ovarian testosterone production as serum DHEA-S and DHEA, also falls with increasing age [68]. Thus, after menopause, androgen insufficiency occurs, in part due to contributions from reduced synthetic function in both the adrenal and the ovaries. Furthermore, SHBG increases in postmenopausal women, especially in women who are treated with oral estrogen therapy [46, 47]. The net effect of a diminished androgen synthesis and an increase in SHBG is a reduced amount of free unbound testosterone. Low androgens are associated with decreased sexual interest, impaired sexual functioning including decreased lubrication, muscle wasting, osteoporosis, loss of energy, changes in mood, and depression [81–83].
A series of investigations [84–87] have found correlations with diminished levels of androgens and female sexual dysfunctions, including sexual pain [87]. Correlations between total testosterone, free testosterone, and DHEA-S were found in one study using the Female Sexual Function Index [88]. A significant correlation between androgens, sexual desire, and sexual arousal was demonstrated in a study of perimenopausal women [84]. Braunstein and colleagues found a significant correlation between sexual desire and total testosterone, as well as with free testosterone, bioavailable testosterone, and dihydrotestosterone [85]. Finally, in a small group of healthy premenopausal women with and without symptoms of sexual dysfunction, those who had sexual disorders had a significant decrease in the concentrations of δ5-androgenic steroids, predominantly in the adrenal gland [86]. However, Davis and colleagues were unable to demonstrate a correlation between total testosterone plasma levels and symptoms of sexual function [89].
There are several relevant clinical presentations associated with low testosterone levels. The one most relevant to premenopausal women with dyspareunia is the use of oral contraceptives [36–40, 47, 48]. In women on oral contraceptives, there is suppression of ovarian testosterone production and increased SHBG synthesis. This combination of effects leads to low calculated free testosterone values. Hyperprolactinemia, which may occur either secondary to a prolactinoma or to psychotropic medications, may result in hypogonadotrophic hypogonadism which causes reduced testosterone and sexual dysfunction. Adrenal insufficiency may result in diminished DHEA sulphate and total testosterone values. Cushing’s disease, or endogenous or exogenous glucocorticosteroid excess, may lead to adrenal suppression and androgen insufficiency. Hyperthyroidism can raise SHBG, thus reducing unbound, free testosterone values [90, 91].
A multinational expert panel assessed the role of androgen insufficiency in women with sexual health concerns, including sexual pain [83]. Androgen insufficiency was defined as a pattern of characteristic clinical symptoms in the presence of decreased bioavailable or free testosterone. These symptoms include a diminished sense of well-being or dysphoric mood, persistent unexplained fatigue, changes in sexual function (including decreased libido, sexual receptivity, and pleasure), vasomotor instability, and decreased vaginal lubrication (even with adequate estrogen treatment). The inclusion of inadequate lubrication in the constellation of androgen insufficiency symptoms makes this especially important to women with sexual pain [83].
Androgens: Clinical Data
Administration of DHEA to women has been shown to increase testosterone levels. One placebo-controlled randomized clinical trial involved a 4-month crossover design of 24 women with adrenal insufficiency [92]. Compared to those subjects who received 50 mg/day of DHEA versus placebo, the active drug increased serum testosterone from below normal to the normal range and significantly increased physical satisfaction. While this trial did not specifically measure sexual pain, this author’s clinical experience is that women with sexual pain improve on DHEA therapy [93]. A retrospective open-label study of DHEA treatment (50 mg/day) in 113 healthy women with sexual dysfunction noted significant increases in lubrication and satisfaction compared to baseline function [93]. Baulieu et al. reported that DHEA treatment doubled total testosterone values and also significantly increased sexual activity and sexual satisfaction after 12months[94].
Several clinical studies have examined the relationship between serum testosterone levels and sexual activity. In two studies of premenopausal women [95, 96], transdermal testosterone therapy significantly improved many aspects of sexual function and behavior including sexual motivation, fantasy, frequency of sexual activity, pleasure, orgasm, and satisfaction. In postmenopausal women [56, 85, 97–99], transdermal testosterone patches significantly increased free testosterone, bioavailable testosterone, and dihydrotestosterone from the lower limit of the normal range to higher values within the normal range.
The frequency of sexual activity and pleasure was significantly greater than placebo in one study, and in another, significant increases were noted in total satisfying sexual activity, arousal, orgasm, pleasure,and body image following testosterone transdermal patch versus placebo in post-menopausal women. Women assigned to testosterone also reported a significant decrease in distress related to sexual function. The symptom improvements in sexual function were considered clinically relevant [98]. Improvement in sexual pain was not specifically noted as the presence of clinically significant sexual pain was an exclusion criterion in these studies. Goldstein and Burrows have reported that administration of testosterone to women with androgen insufficiency syndrome and sexual pain is associated with symptom improvement [100].
Any woman with sexual pain treated with androgen therapy needs to be thoroughly counseled regarding risks and benefits and the need for routine follow-up and blood test surveillance testing [101]. Safety issues for DHEA and androgen administration include acne and hirsutism [56, 85, 92–94, 97–99, 101]. Side effects such as balding, voice deepening, cliteromegaly, and polycythemia were not noted in clinical trials. There was no evidence that exogenous testosterone increases the risk of endometrial cancer or endometriosis. No significant adverse effects were noted from baseline on measures of blood lipids, including total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, or triglycerides [56, 85, 92–94, 97–99, 101].
Estrogens/Progestins: Physiology and Pathophysiology
Throughout the reproductive years, the primary source of estradiol is cyclical synthesis by the ovaries, which are under the control of the pituitary via FSH and inhibin. There is a rise in estradiol in the late follicular phase of the menstrual cycle and in the luteal phase. The luteal phase is characterized by a rise in progesterone, whose primary source of synthesis is the corpus luteum of the ovary. As long as the premenopausal woman continues to regularly ovulate, estrogen and progesterone levels are maintained until the time of menopause. When ovulation ceases at the time of menopause, estradiol and progesterone levels fall abruptly. Outside of the ovary, estrogen synthesis occurs via adrenal and ovarian androgen precursors. In addition, estrogen continues to be synthesized in the periphery (e.g., skin, adipose tissue, bone, muscle) in postmenopausal women through conversion of androstenedione to estrone and testosterone to estradiol, but the amount of estradiol synthesized depends, in part, on the enzymatic activity of aromatase.
Premenopausal women with sexual dysfunction, including dyspareunia, who do not have regular, normal menstrual cycles and are otherwise amenorrheic, dysmenorrheic, or menorrhagic, should have the underlying pathophysiology managed. Other medical issues during the premenopausal years that interfere with cyclical estrogen and progesterone production include rapid weight loss and anorexia nervosa [102]. It has been well documented that estrogen and progesterone levels fall in these conditions and women may exhibit sexual dysfunction including dyspareunia.
Estrogens and other sex steroids act via cytosolic receptors in a genomic process that utilizes transcription to induce protein synthesis directed to specific central nervous system or peripheral genital tissue structure and function. Estradiol acts in a nongenomic fashion with direct interactions with numerous central neurotransmitter systems including catecholaminergic, serotonergic, cholinergic and Г-aminobutyric acidergic systems [103]. The high concentrations of estradiol in the hypothalamus and the preoptic area suggest that it is involved in sexual behaviors [104, 105].
Less is known about the relationship of progesterone with sexual function [105–110]. Progesterone has ge-nomic activity via progesterone receptors that modulates gene expression and thus regulates neuronal networks that control sexual behavior. Of note, estradiol increases the expression of progesterone receptors that in turn function as a critical coordinator of sexual response [105-110].
Estrogens/Progestins: Clinical Data
Should there be the need for exogenous estrogen and progesterone, there are many treatment options. Bioidentical and synthetic forms of estradiol and progesterone are available via multiple delivery systems including oral, transdermal, transvaginal, or parenteral. The choice of estrogen and progesterone utilized (synthetic or nonsynthetic) may have important implications on the woman’s sex steroid hormonal milieu, especially SHBG and androgen values. Thus, the choice of estrogen and/or progesterone may adversely influence sexual function. This is especially pertinent for synthetic progestin agents [111].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree