Fig. 15.1
A 43-year-old blunt trauma patient presenting with hypotension. Tension pneumothorax was not clinically diagnosed as the cause of hypotension and this chest radiograph was obtained. The image shows left-sided pneumothorax with mediastinal shift to the right causing vena cava compression and life-threatening hypotension. The patient’s vital signs normalized after left-sided needle thoracostomy. A chest tube was subsequently placed. Image courtesy of Dr. Timothy Emhoff, University of Massachusetts Medical School
The teenager or young adult with a spontaneous rupture of a congenital bleb can usually identify the onset of acute pain. As these individuals are healthy, they usually complain of minimal to no dyspnea [6–8]. They are rarely hypoxic. Conversely, the middle-aged smoker with COPD and rupture of an emphysematous bleb will usually be dyspneic due to the acute loss of lung volume on the background of borderline pulmonary function. Physical examination will reveal absent breath sounds on the affected side. Auscultation should always be performed in the axilla as transmitted contralateral breath sounds may be falsely interpreted as ipsilateral aeration. The examiner may note chest wall tenderness and crepitus due to subcutaneous emphysema. This occurs when air leaking from the lung insinuates into the chest wall through a tear in the parietal pleura. It is classically manifest on exam as “Rice Krispies crunching” under the skin, and, if widespread, may cause the patient to appear like the “Stay Puft marshmallow man” due to subcutaneous emphysema involving the neck and face.
The diagnosis of PTX in the trauma setting has traditionally been made on a supine anterior–posterior (AP) chest radiograph [9]. Recently the extended focused assessment with sonography for trauma (FAST) exam has been promoted as a more sensitive modality for the identification of PTX, especially smaller ones. A recent meta-analysis revealed that ultrasound was 86–97% sensitive for detecting traumatic PTX as opposed to 28–75% for supine chest radiograph [9, 10]. The normal chest ultrasound will show visceral and pleural surfaces “sliding” over one another during respiration. Absence of “sliding” is a sensitive and specific indicator of PTX. In some trauma centers, ultrasound has supplanted initial supine radiograph for the diagnosis of PTX [9, 11]. Stable trauma patients without concern for spine injury should have an upright posterior–anterior (PA) chest radiograph. Many smaller PTX in trauma are seen only on computed tomography (CT) scan of the chest. These are termed occult PTX. Stable patients suspected of a non-traumatic PTX should still have conventional upright PA and lateral radiographs in the radiology suite.
Because it is immediately life-threatening, the conventional teaching for tension PTX is that if it is suspected on clinical grounds, it should be treated without confirmatory radiography [12]. The increasing availability of immediate bedside ultrasound may alter this practice in the future as more information on the efficacy of this modality emerges [13, 14].
Management
All patients who are being seen in consultation for a traumatic chest injury should have an appropriate overall trauma evaluation commensurate with the Advanced Trauma Life Support (ATLS) practices of the American College of Surgeons and in accordance with local institutional practices [12]. The traditional initial treatment for all symptomatic traumatic PTX has been, and for the most part remains, the placement of a large tube thoracostomy tube (32–36 French) in the fifth intercostal space at the anterior or mid-axillary line with the application of suction through a water seal drainage system [12]. Recently, however, there has been a trend to place smaller percutaneous tubes by the Seldinger technique in the second intercostal space at the anterior axillary line for the evacuation of smaller PTX. Though seemingly less traumatic, the use of these so-called pigtail catheters remains controversial. Small amounts of concurrent pleural blood, often unappreciated on semi-upright chest radiographs after injury, may clog these tubes. Fig. 15.2a shows a subtle left-sided HTX as visualized on the initial chest radiograph taken in the trauma bay. However, as seen in Fig. 15.2b, the patient had a sizeable posteriorly layering left HTX. Additionally, pigtail catheters do not have the diameter to evacuate large-volume air leaks and often require a second tube [15].
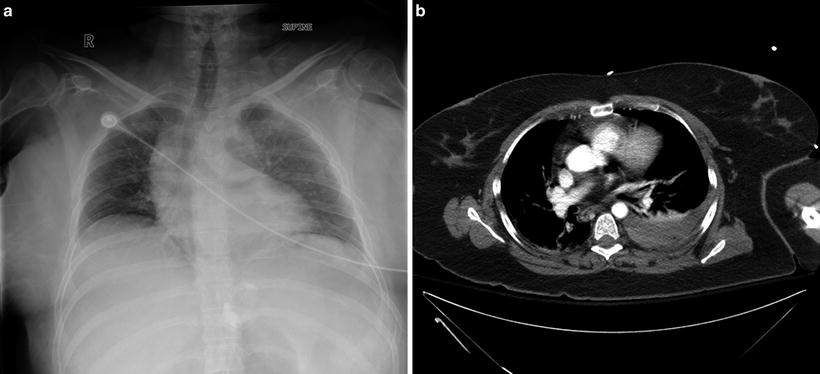

Fig. 15.2
(a) This supine radiograph shows slightly increased opacity on the left side. The size of hemothorax was not appreciated until subsequent CT imaging was obtained. (b) This CT scan of the chest corresponds to Fig. 15.2a and shows a sizeable left-sided hemothorax that was missed on initial supine chest radiograph. Images courtesy of Dr. Dennis Coughlin, University of Massachusetts Medical School
Patients who have hemodynamic or respiratory instability felt due to a tension PTX should have needle decompression of the pleural space using a 14 gauge 2-in. angiocatheter in the second intercostal space at the mid-clavicular line while preparations are made for thoracostomy tube placement. This is an immediate life-threatening condition and should be diagnosed on clinical grounds rather than radiographic findings as demonstrated in Fig. 15.1. Decompression will release positive pressure, allowing the mediastinum to return to midline and the patient to re-compensate. If the diagnosis of tension PTX was correctly made, the patient will improve in color and blood pressure almost immediately. A tube thoracostomy is then required as previously mentioned to treat the simple (non-tension) PTX thus created [12, 16].
Once the desired thoracostomy tube is placed, lung inflation should be confirmed by chest radiograph, preferably upright, and the amount of air leak, if any, should be noted. Failure of the lung to inflate with a large continuous air leak in the water seal chamber may indicate a tracheobronchial injury, which should be confirmed by bronchoscopy. If present, a second chest tube should be considered as the first maneuver. Thoracic specialty consultation should be obtained. The management of this injury is beyond the scope of this text. Failure of the lung to inflate without an air leak indicates mal-positioning of the tube, usually in the subcutaneous tissue or occlusion of the chest tube, drainage system, or bronchi. The system should be checked and bronchoscopy may be indicated. After placement of a thoracostomy tube, trauma patients treated for PTX should have a dynamic chest CT or CT angiogram contemplated to assess other latent thoracic injuries. It is well recognized that three thoracic injuries are identified on CT for every single injury noted on chest radiograph.
The increased sensitivity of CT scans will document occult PTX. Occult PTX is defined as a PTX noted only on CT scan but not evident on conventional chest radiograph [2, 17–20]. There is an increasing body of evidence and expert opinion that occult PTX are extremely unlikely to enlarge and can therefore be managed conservatively, as long as they remain stable in size and patients remain free of respiratory symptoms attributable to the PTX [13, 21]. Some have reported the successful deferral of thoracostomy even in the setting of positive pressure ventilation for surgical procedures [22]. However, the decision to defer thoracostomy should be approached cautiously in the multi-trauma patient. In this setting, an untreated PTX may create confusion when the patient suffers a hemodynamic or respiratory decompensation for unrelated reasons.
The spontaneous PTX due to congenital bleb in the young patient may be treated with a tube thoracostomy in the second interspace at the midclavicular line using a conventional tube or a pigtail catheter. The COPD patient with emphysematous bleb rupture may be treated similarly. However, in this situation a non-contrast chest CT should generally be obtained prior to tube placement (assuming that the patient is stable without evidence of tension physiology or respiratory compromise) to differentiate PTX from a giant emphysematous bleb [6–8, 23]. Placement of thoracostomy tubes into giant emphysematous blebs results in a bronchopleural fistula, which is very difficult to manage.
Small iatrogenic PTX due to attempted subclavian central venous catheter placement can generally be treated by observation or by placement of an apical pigtail catheter with good results since the site of injury is known to be at the apex. Larger iatrogenic PTX should receive conventional thoracostomy tubes placed in the apical position [24]. Table 15.1 presents pearls for thoracostomy tube placement.
Table 15.1
Tube thoracostomy placement procedure
“Pearls” for tube thoracostomy placement |
|---|
•Enter pleural space using a blunt Kelly clamp controlled by two hands •Place index finger in pleural space prior to tube placement to assess for adhesions •Place the tube attached to clamp. “Free hand” placement always fails with tube residing in subcutaneous tissue •Fix the tube with “0” suture material, silk, braided polyester, or equivalent. Slightly indent tube with suture to assure firm hold •Failure of lung to inflate without air leak means system obstruction or bronchial obstruction •Failure of lung to inflate with large air leak may indicate central tracheobronchial injury. Second tube and/or bronchoscopy indicated |
Once the chest tube is in place and inflation of the lung is confirmed on radiograph, numerous protocols and guidelines exist for tube management [25]. In general, once the lung is inflated and pleural surfaces are brought into apposition, the air leak should cease. The thoracostomy tube is then left to suction for 24–48 h to allow pleural symphysis or sealing to occur. Time to sealing will vary with the magnitude of the air leak and the patient’s overall nutritional status. The removal of the tube from suction, known as placement on “water seal,” is generally used to confirm healing of the pleural leak. The water seal chamber on the drainage system is monitored for recurrence of an air leak, and if none is noted a confirmatory radiograph is done, typically from 3 to 8 h later. However, there is very little evidence supporting the duration of time necessary on water seal before a radiograph is performed [26]. If a recurrent leak is noted in the drainage system leak chamber, the system is placed back on suction and a radiograph is done to confirm that the lung remains inflated. Suction is continued another 24–48 h before reattempting water seal.
Importantly, the lung may remain inflated on water seal even when small air leaks are present since a route of egress for the air is available. Removal of the tube in this setting will lead to a recurrent PTX. Therefore, it is important to identify even small leaks in the leak chamber of the drainage system. This can be done by checking for air leaks during forced expiration by asking the patient to take deep breaths in and out and to cough. If a forced expiratory air leak is identified the chest tube should remain in place until completely resolved.
As noted previously, failure of the lung to inflate after tube thoracostomy with a large air leak should raise concern for tracheobronchial injury. However, persistent failure of small air leaks to seal despite inflated lung or recurrent PTX on water seal may present both a diagnostic and therapeutic challenge. A CT scan with the thoracostomy tube on suction should be done to identify uninflated areas of lung that may be contributing to a persistent air leak. Additional thoracostomy tubes or radiologically guided drainage catheters may be required. Video-assisted thoracoscopic surgery (VATS) for pleurodesis may ultimately be needed [27–30]. Surgical pleurodesis involves the application of various irritants to the pleural surfaces to cause inflammatory adhesion of the visceral and parietal pleura and thereby seal air leaks. There is some literature advocating very early VATS intervention in persistent air leak in trauma patients at 48 h post injury [31, 32]. As of this writing, any benefit of such an approach for PTX alone has not been definitively demonstrated. Figure 15.3 shows a general composite algorithm for the management of traumatic PTX.
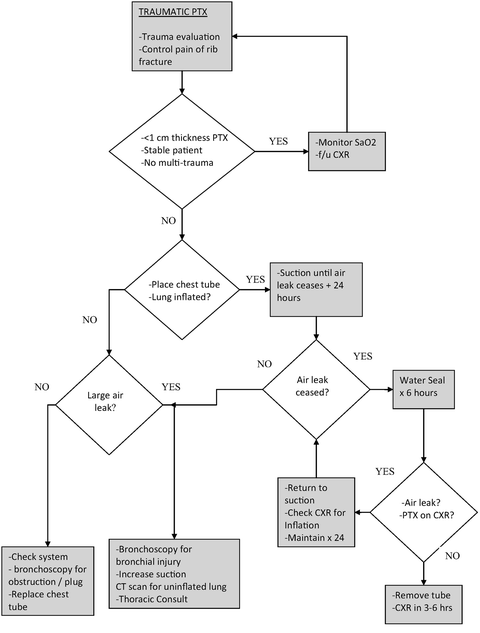

Fig. 15.3
Algorithm for the management of acute traumatic pneumothorax
Importantly, with spontaneous PTX due to ruptured congenital or emphysematous blebs, the lungs are inherently abnormal and sealing of the air leak may take longer than 48 h or may even fail to occur. When tube thoracostomy fails to seal a pleural leak in this clinical setting, surgical intervention, typically VATS with pleurodesis, is required.
Complications
The most common complication of PTX is respiratory failure. In the trauma setting, this is usually due to the combined effect of the loss of lung volume, pain, and splinting from associated rib fractures, burden of associated injuries, and any preexisting pulmonary conditions. Consequently, in addition to reinflating the lung, a thorough approach must be taken in ameliorating the concomitant causes of respiratory failure in the trauma patient. Failure of lung inflation is a rare complication of conservative management without thoracostomy. This usually does not occur unless there is associated pleural blood (HTX) causing an inflammatory response and resultant trapped lung (further described in the next section).
The main iatrogenic complications of thoracostomy placement include injury to almost any intrathoracic structure. This can be avoided by carefully palpating and exploring with the operating finger through the thoracostomy incision prior to placing the tube. Adhesions are swept away and placement above the diaphragm is ensured. Often chest tube placement introduces some degree of additional pain and immobility to patients which is not desirable. Consequently, the decision to place a tube should be carefully considered in cases of smaller PTX. Reasonable respiratory benefit should be expected as a trade-off for the potential increase in splinting due to the presence of the tube.
Follow-Up
There is no evidence that patients who have had traumatic PTX inflated by a thoracostomy and show full inflation on “post-pull” radiograph several hours later need any further imaging as long as they remain clinically well. Nonetheless, it is the customary practice at many institutions to obtain a follow-up radiograph prior to discharge or at clinic visit. Patients who have been treated conservatively for small PTX generally receive a follow-up radiograph several weeks later to document full lung expansion, though the benefit of this also remains unclear. Recurrences are rare in otherwise healthy individuals in either situation. There is no standardized follow-up for patients with spontaneous PTX due to congenital bleb or emphysematous bleb rupture. These patients will generally become symptomatic if recurrences occur. They should maintain a close relationship with their pulmonologist or thoracic surgeon as recurrence rates may be as high as 50% [6–8].
Hemothorax
Epidemiology
The single major cause of HTX for all age groups is trauma, usually blunt [33]. Twenty-five percent of patients with chest trauma will be diagnosed with HTX [34]. Here the typical etiologic factor is intercostal vessel bleeding caused by fractured ribs, though other sources such as lung parenchyma laceration or hilar vascular injuries have been reported. Rare non-traumatic causes include pleural malignancy, iatrogenic injury, and spontaneous HTX due to pathologic coagulopathy [35]. For the purposes of this discussion we will be referring to traumatic HTX unless otherwise specified.
Clinical Presentation and Diagnosis
Three initial (acute) clinical presentations are common for HTX, each requiring its own approach to management. These include (1) immediate HTX with hemodynamic instability, (2) immediate HTX without hemodynamic instability, and (3) delayed HTX, which may appear up to 2 weeks after chest wall injury in up to 7% of all patients with rib fractures [33, 36, 37].
The unstable patient presenting to the trauma bay will have an appropriate history of mechanism, for example motor vehicle driver with side impact or stab wound to the thorax or upper abdomen. They may be hypotensive or merely tachycardic. Oxygen saturation may be low or normal depending upon loss of lung volume, other injuries, and preexisting respiratory status. They may complain of dyspnea. Physical examination reveals absent breath sounds on the affected side. Here, diagnosis is often made by emergent placement of the thoracostomy tube and the expression of a large amount of blood.
The stable patient with HTX may have respiratory or pain-related complaints or both. Clinical examination may or may not identify decreased breath sounds but there will often be chest wall tenderness. Here diagnosis is made by chest radiograph, preferably in the semi-upright position. It should be remembered that on the supine radiograph, very significant fluid collections may yield only a barely detectable increased opacity on the injured side (Fig. 15.2). These HTX may not be suspected until incidentally noted on the lower thoracic cuts of an abdominal CT. Several centers have begun to utilize the FAST examination to identify HTX, but the efficacy of this has not yet been fully determined [38].
The patient presenting with a delayed HTX is often in-hospital or may even have been discharged to home, as this acute bleeding may occur up to 2 weeks after initial trauma [39, 40]. The mechanism here is hypothesized to improve pain control allowing for increased respiratory excursion that then results in a new tear of an intercostal vessel at a fracture site. The incidence of delayed HTX is increased with multiple and displaced rib fractures. Presentation is varied and may be predominantly one of blood loss with tachycardia, malaise, or overt hemorrhagic shock. Alternatively, it may manifest as respiratory symptoms ranging from mild exercise intolerance all the way to respiratory distress. Presentation may be very subtle and missed in the patient still incapacitated from distant injuries. For patients on mechanical ventilation, the HTX may be a surprising incidental finding on a routine chest radiograph.
Management
In severe or multi-trauma patients, the treatment of HTX, like that of any injury, should be prioritized based on the principles of ATLS [12]. If an HTX is suspected or documented on chest radiograph, a large thoracostomy tube, 36 or 40 French, is inserted in the fifth intercostal space at the posterior or mid-axillary line. If a massive HTX is expected based on radiograph or presentation, an autotransfusion attachment should be placed in line with the chest drainage system to scavenge and return shed blood. This may provide an immediate improvement in stability; however, it has not been shown to decrease transfusion requirements [36].
For the unstable patient whose immediate chest tube output is ≥1,500 ml, a massive HTX, and who remains unstable, immediate thoracotomy remains the treatment of choice along with autologous and banked blood resuscitation [12]. This has been based on the rationale that large shed blood volumes indicate injury to larger vessels, such as intercostal arteries, the internal mammary artery, or more central lung vasculature that are less likely to cease bleeding without operative control of the vessel in question. For patients with large initial thoracostomy outputs who are stable, or readily stabilized, VATS is being used successfully in the acute setting by several groups to avoid thoracotomy [31, 41]. It has been shown that this procedure can be safely performed by acute care surgeons with the appropriate experience with thoracic surgical backup as needed [42]. Electrocautery and clips can be applied thoracoscopically to control moderate hemorrhage. The lower morbidity of this procedure may make it applicable in the future to patients with lesser but still significant thoracostomy outputs for whom the risk/benefit ratio for full thoracotomy may be unfavorable.
Stable patients with lesser immediate chest tube output should have complete evacuation of the HTX with tube thoracostomy and subsequent lung inflation documented on an upright chest radiograph. The trauma workup should be completed as necessary. This should include chest CT to identify significant thoracic injuries related or unrelated to the HTX, especially in the setting of penetrating trauma. The chest tube output is monitored hourly and the patient monitored in at least an intermediate care setting. Numerous protocols exist to trigger surgery in such stable patients based on continuing chest tube output. Examples are 250 ml/h for 2 h or 125 ml/h for 4 h [12, 37]. These are not hard-and-fast rules, however, and there is no substitute for good surgical judgment. Factors to consider when deciding upon intervention, be it by thoracotomy or VATS, include trend in chest tube output, patient stability, associated injuries, and the patient’s overall health. Sudden cessation of chest tube output during monitoring, particularly if the patient becomes unstable, should raise concern for occlusion of the thoracostomy tube and prompt an immediate chest radiograph to assess for re-accumulation.
Some success has been reported using angiographic embolization to control intercostal bleeding or internal mammary artery bleeding in stable patients for whom chest CT has shown contrast extravasation or a “blush” indicative of arterial bleeding [43]. More validation is required for use of this modality, but over time this may prove applicable to stable patients with moderate thoracostomy output. Figure 15.4 shows a patient successfully treated with angioembolization of the left internal mammary artery for HTX sustained due to a fall.
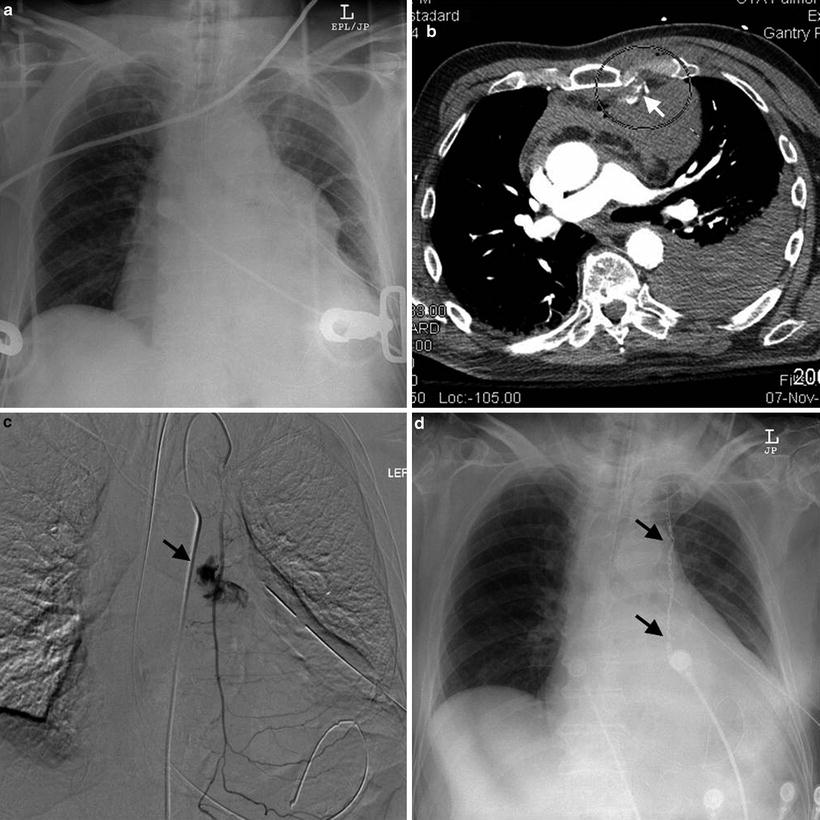

Fig. 15.4
An 88-year-old blunt trauma patient with sterna and rib fractures transferred to trauma center after left-sided chest tube placement for hemothorax. (a) Shows initial chest X-ray. White arrow in (b) shows contrast extravasation from the left internal mammary artery adjacent to bone fragments from the sterna fracture (circled). Dark arrow in (c) shows ongoing contrast extravasation at angiography. The patient was successfully treated with coil embolization as shown by the dark arrows in (d). Image courtesy of Dr. Suvaraju Ganguli and Ms. Stephanie Hanson, Massachusetts General Hospital
Patients with lesser initial chest tube drainage (<1,000 ml) and clearing of the chest radiograph who remain unstable should be investigated for extrathoracic injuries or non-traumatic causes of their shock. Figure 15.5 illustrates a composite of algorithms for the management of acute HTX.
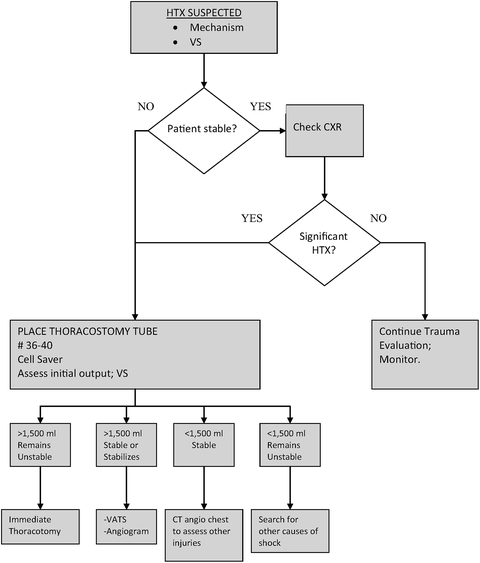

Fig. 15.5
Algorithm for the management of acute traumatic hemothorax
In stable patients with radiographic evidence of HTX, the question commonly arises as to how much volume of intrathoracic blood mandates drainage. Several studies have indicated that complications can occur with as little as 300–500 ml of intrathoracic blood [44]. In general, almost all traumatic HTX that completely opacify the costophrenic angle on plain radiographs should be evacuated as early as possible to prevent complications [37, 42]. In multi-planar CT imaging, this has been correlated to the thickness of the lateral pleural fluid stripe. Evidence suggests that a stripe >1.5 cm in thickness is an indication for a drainage intervention [45]. Table 15.2 illustrates pearls for the management of acute traumatic HTX.
Table 15.2
Management of acute traumatic HTX
“Pearls” for management of acute traumatic hemothorax
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|




