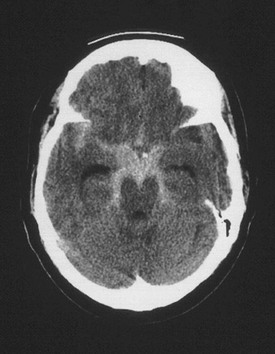
Chapter 103 Headache is a common complaint, accounting for approximately 3 million visits to the emergency department (ED) per year in the United States.1 In addition, many more patients present with headache as part of a constitutional illness, making the symptom of headache one of the most frequent complaints in the ED.1 Headache commonly is divided into primary and secondary disorders.2 The primary headache disorders include migraine, cluster, and tension-type headaches, which represent the majority of headaches seen in clinical emergency practice.3–5 Secondary headache disorders include a variety of organic illnesses in which head pain is a symptom of an identifiable, distinct pathologic process. To facilitate a standardized approach to headache management, the International Headache Society published a classification system and diagnostic criteria for headache disorders, cranial neuralgias, and facial pain.2 This comprehensive and widely accepted system includes 14 major categories of headache disorders and uses specific operational diagnostic criteria to define each headache type (Box 103-1). Most patients presenting to an ED with headache have a benign primary headache disorder requiring only symptomatic treatment and referral. The challenge for the emergency physician is to identify the very small subset of patients who have headache as a symptom of a serious or potentially life-threatening disease. Migraine is a common, chronic, sometimes incapacitating neurovascular disease characterized by recurrent attacks of severe headache, autonomic nervous system dysfunction, and, in some patients, an aura causing visual, sensory, motor, or other neurologic symptoms.6 It is a primary headache disorder with a genetic basis.7 Migraine headaches account for approximately 1 million visits to the ED per year.8 Migraine attacks typically begin in the second decade of life and peak in prevalence in the fourth decade of life, with a 1-year period prevalence of 7% of men and 24% of women.9 Overall, migraine is more prevalent among women (18%) than among men (6%).9 During childhood, however, there is no gender difference in the prevalence of migraine. After menarche, a correlation between migraine and menstruation is noted in half of female migraine sufferers. After menopause, the prevalence of migraine among women decreases.10 Historically, migraine headaches have been considered to be vascular in origin. However, this hypothesis is no longer tenable as alterations in cerebral blood flow do not correlate with the various phases of the headache attack or vascular territories and do not explain features of an acute migraine such as premonitory mood disturbances, nausea, and osmophobia. Rather, vascular changes are now thought to be an epiphenomenon to what is a primary neurologic event.11 Abnormal trigeminal nerve activation, possibly triggered by cortical spreading depression or, less likely, a sterile neuropeptide-induced inflammatory process, leads to pain and sensitization of higher order neurons in the brainstem and thalamus. Descending modulation is likely to be compromised as well. It is not yet known what initiates the pathophysiologic process that leads to a migraine attack. Migraine is commonly thought of in two major categories: migraine without aura, or common migraine, which is the most frequent form of migraine and accounts for approximately 80% of all cases (Box 103-2); and migraine with aura, or classic migraine, which has specific reversible neurologic symptoms that precede the actual headache (Box 103-3) and is seen less frequently.6 The aura of classic migraine consists of focal neurologic symptoms that precede and herald the migraine attack. By definition, the aura is fully reversible and typically lasts 10 to 20 minutes, although it may continue for as long as 1 hour. The most common aura is visual; features may include scintillating scotomas (bright rim around an area of visual loss), teichopsias (subjective visual image perceived with eyes open or closed), fortification spectrums (zigzagged wall of fortress slowly drifting across visual field), photopsias (poorly formed brief flashes or sparks of light), and blurred vision. Less common auras include somatosensory phenomena such as tingling or numbness, motor disturbances, and cognitive or language disorders.6 Ophthalmoplegic migraine is a rare syndrome associated with paresis of one or more ocular nerves, most commonly the third cranial nerve. Patients typically present with ipsilateral headache associated with extraocular muscle paresis and occasionally pupillary changes. The ophthalmoplegia or pupillary changes may last for days to weeks and rarely may become permanent. Because of the neurologic abnormalities, secondary causes including intracranial aneurysm and mass lesion should be ruled out in the absence of a clear history of recurrent identical episodes.12 Hemiplegic migraine is characterized by a motor aura consisting of hemiparesis or hemiplegia. The progression of the motor deficit is gradual and in most cases is accompanied by a visual, sensory, or speech disturbance. The neurologic symptoms last up to 60 minutes, followed by headache. Rarely, the motor deficit is persistent, resulting from a true migrainous stroke.2 A familial version of hemiplegic migraine is associated with genetic mutations of ion transporters.7 Basilar-type migraines arise with an aura referable to the brainstem and are associated with multiple neurologic findings, including visual symptoms (often total blindness), dysarthria, tinnitus, vertigo, bilateral paresthesias, paresis, and altered level of consciousness. The symptoms are stereotypic and resolve spontaneously.2 Many factors can trigger migraine headaches in predisposed persons. Common precipitants include sleep deprivation, stress, hunger, hormonal changes including menstruation, and use of certain drugs including oral contraceptives and nitroglycerin.13 In addition, some patients report specific food sensitivities to chocolate, caffeine, and foods rich in tyramine, monosodium glutamate, and nitrates. Alcohol, specifically red or port wine, has also been implicated. In others, certain sensory stimuli, such as a strong glare or strong odors, loud noises, and weather changes, can trigger an attack.6 Routine neuroimaging is not necessary for patients with typical recurrent migraine headaches.14 An increasing appreciation of medical imaging–induced radiation toxicity warrants prudent use of computed tomography (CT) scanning in younger patients. Neuroimaging should be considered for older or immunocompromised patients with new-onset headaches, headaches with a progressive course, headaches worsened by Valsalva maneuver, headache causing awakening from sleep, or headaches associated with unexplained neurologic abnormalities.14 Such patients have a higher likelihood of having a secondary cause of headache, such as tumor, arteriovenous malformation, or structural lesion. In addition, patients who present with a thunderclap or abrupt-onset headache require a lumbar puncture to rule out subarachnoid hemorrhage if findings on a CT scan are normal.15 The description “worst headache of life” may be associated with subarachnoid hemorrhage but suffers from interobserver variability.16 The pharmacologic treatment of migraine is divided into abortive therapies, which attempt to limit the intensity and duration of a given episode, and prophylactic therapies, which are intended to decrease the frequency and intensity of attacks. The goals of acute migraine therapy include rapid pain relief, minimization of headache recurrence and medication side effects, restoration of the patient’s ability to function, and minimization of the use of backup and rescue medications.17 There are several approaches to treatment of the acute headache episode, depending on the severity of the attack (Table 103-1). The choice of agents depends on the patient’s previous response to specific therapies, the existence of comorbid conditions, and the presence or absence of nausea or vomiting. Gastric stasis is common during acute migraine attacks and may limit the effectiveness of oral agents.18 For mild to moderate attacks, simple analgesics such as acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs) are often effective.19,20 In the presence of nausea or vomiting, the addition of an agent such as metoclopramide enhances the absorption and effectiveness of these medications.21 Appropriate doses and possible side effects are listed in Table 103-1. Dopamine antagonists, such as the neuroleptics prochlorperazine and chlorpromazine, and the antiemetics metoclopramide and droperidol have been shown to be highly effective as monotherapy for acute migraine attacks.22–24 Because of their efficacy, safety, tolerability, and few contraindications, they are used by many emergency physicians as first-line therapy for many migraine patients. For this class of medication, clinical research has outpaced preclinical work, and thus a compelling mechanism of action is lacking. However, migraine pathogenesis is likely to involve dopamine pathways.25 The most common side effects after parenteral administration include sedation, postural hypotension, and extrapyramidal symptoms, most notably akathisia, which can be treated with diphenhydramine or midazolam.26 DHE is administered intravenously (IV) in a dose of 1.0 mg during 2 minutes; this can be repeated in 1 hour if pain control has not been achieved. Because DHE can cause nausea and vomiting, patients should be pretreated with an antiemetic such as metoclopramide 10 mg IV or prochlorperazine 10 mg IV. Repeated administration of the intravenous form of DHE is effective in patients with status migrainosus.27 Contraindications to use of DHE include pregnancy, breast-feeding, poorly controlled hypertension, coronary artery disease, and peripheral vascular disease. DHE should not be used if the patient has already taken any drug in the triptan class or if the patient is using macrolides or protease inhibitors. Sumatriptan, the first-approved medication of the triptan class, a class of selective 5-HT (1B/1D) receptor agonists, is available for subcutaneous administration, and it is the most common triptan preparation used in the ED setting.8 Other triptans available in the United States include eletriptan, almotriptan, zolmitriptan, naratriptan, frovatriptan, and rizatriptan. The initial dose of sumatriptan is 6 mg given subcutaneously, which may be repeated once in 1 hour if the patient has a partial response to the first dose. Common side effects include tingling, flushing, warm or hot sensations, heaviness in the chest, and initial worsening of the underlying headache.28 Sumatriptan has contraindications similar to those for DHE and should not be used within hours of administration of an ergotamine-containing medication or DHE. A smaller dose of subcutaneous sumatriptan may limit side effects.29 Opioid analgesics such as morphine should be reserved for patients who do not respond to any of the medications listed or have contraindications to all standard migraine therapies. Although opioids are frequently used, they have been shown to be less efficacious than other agents and are associated with frequent ED visits.30,31 Recurrence of migraine within 24 hours of ED discharge is common, regardless of medication administered or pain intensity at discharge.32 Corticosteroids are effective at decreasing the rate of migraine recurrence after ED discharge, although with a number needed to treat (NNT) of 9, these medications are not necessarily indicated for all patients.33 Oral naproxen 500 mg and sumatriptan 100 mg are reasonable treatment options for the recurrent headache.34 Prophylactic therapy is indicated for patients who have frequent attacks (more than two or three functionally disabling episodes per month), prolonged attacks lasting more than 48 hours, or attacks that are severe and debilitating. Several classes of medications are used for the prophylaxis of migraine, including beta-blockers, tricyclic antidepressants, and antiepileptic drugs.35,36 Prophylactic therapy is best initiated in consultation with the patient’s primary care provider or neurologist. Cluster headache is the only headache syndrome that is more common in men than in women. It typically occurs in young to middle-aged adults who smoke, with a peak incidence in the late 20s.37 The headaches tend to occur repeatedly during a defined time interval, hence the term cluster. Several attacks can occur in 1 day, and a typical cluster period may last weeks to months. Several precipitating factors have been implicated, most notably the ingestion of alcohol. Stress and climatic changes may also play a role in susceptible persons. Cluster headaches occur suddenly with little warning, and multiple episodes can occur within a 24-hour period. Each headache lasts from 15 minutes up to 3 hours. The headache typically begins with a unilateral sharp, stabbing pain in the eye, which may awaken the patient from sleep. The attacks occur exclusively in the territory of the trigeminal nerve.38 Unlike the migraineur, the patient with cluster headache predictably presents agitated and anxious, rocking, rubbing the head, and pacing. The attack subsides rapidly, often leaving the patient exhausted. Accompanying the headache are ipsilateral autonomic symptoms such as ptosis, miosis, and forehead or facial sweating. The eye often is injected and tearing, and many patients have unilateral nasal congestion.2 Other headache disorders that mimic cluster headache include carotid artery dissection, trigeminal neuralgia, and the rare trigeminal autonomic cephalalgia paroxysmal hemicrania. Carotid dissection should be excluded as the diagnosis in patients who present with unilateral face or neck pain and Horner’s syndrome. With trigeminal neuralgia, the pain peaks within seconds, lasts only a couple of minutes, and can be provoked by specific trigger points on the face or oral mucosa. The trigeminal autonomic cephalalgias are manifested by a brief unilateral headache that recurs dozens of times per day, often accompanied by the same unilateral eye and nasal symptoms as cluster.2 Cluster headache is brief in duration and may resolve before a patient presents to medical attention. High-flow oxygen should be considered first-line therapy. Delivered through a non-rebreather mask at a rate of 12 L/min, it has been shown to abort the headache within 15 minutes in 78% of patients, with an NNT of 2 compared with patients who breathe room air.39 Subcutaneous sumatriptan is also an effective therapy for acute cluster headache and should be dosed at 6 mg.40 Larger doses do not confer much benefit, whereas smaller doses may decrease some of the medication side effects. Alternatively, octreotide or intravenous dopamine antagonists may be effective.41,42 Once the acute attack has been relieved, the emergency physician’s work is not done. The acute attack is part of an ongoing “cluster” of headaches that likely will recur the following day. Corticosteroids have long been theorized to help break the cluster, although high-quality evidence is not available. A recommended regimen is 60 mg of prednisone daily for 10 days, followed by a 1-week taper. Verapamil, dosed at 120 mg three times a day, will decrease the frequency of attacks by the end of the first week of therapy.43 Tension-type headache is the most common recurrent headache disorder, affecting more than 50% of the population, but it is an infrequent cause of ED visits.4,44 Women are affected slightly more frequently than men are, with peak prevalence in the fourth decade of life. These headaches typically do not cause substantial functional disability, and patients are able to continue with their normal daily activities. The median frequency of headaches is six per month, and stress and lack of sleep are implicated as triggering factors.2,45 By definition, episodic tension-type headache lasts as little as 30 minutes and as long as 7 days. Little is known about the pathophysiology of tension-type headache. There is no clear evidence that increased muscle activity is present, and the physical examination will reveal tender areas of the scalp and neck with both tension and migraine headaches. Despite different epidemiologic profiles, similar response to many therapeutics suggests that tension and migraine headaches may be part of a continuum with similar pathophysiologic mechanisms.46,47 Patients typically complain of a tight, bandlike discomfort around the head that is nonpulsating and dull. They also may experience tightening of the neck muscles. A majority do not seek medical assistance because the headache usually is mild in intensity and not functionally disabling. On occasion, the discomfort can build up slowly and fluctuate in severity for several days. Unlike in migraine, the headache does not worsen with physical activity, and accompanying symptoms, such as nausea, vomiting, phonophobia, and photophobia, are unusual. Anxiety and depression may coexist with chronic tension headache, which by definition occurs more than 15 days a month and can be daily and unremitting.2 Tension headache is the least distinct of all of the primary headache disorders, and its diagnosis is based mainly on the absence of features that would suggest another headache diagnosis. Because of this lack of specificity, the clinician often may hesitate to make this benign diagnosis without other investigations to exclude organic disease. The most common disorders mimicking tension headache are migraine, idiopathic intracranial hypertension, oromandibular dysfunction, cervical spondylosis, sinus or eye disease, and intracranial masses.48 For a majority of patients with tension headaches, simple analgesics, such as acetaminophen or an NSAID, are adequate for pain control. Because tension-type headache is more common in sedentary persons, a regular exercise program may help.37 Antiemetic dopamine antagonists may be useful therapy.49 Patients with chronic symptoms may exhibit signs of depression or anxiety, and these patients often respond to medications and nonpharmacologic regimens that treat these conditions. Some nonpharmacologic regimens are meditation, massage, and biofeedback. Subarachnoid hemorrhage (SAH) refers to extravasated blood in the subarachnoid space. Presence of the blood activates meningeal nociceptors, leading to diffuse occipital pain along with signs of meningismus. SAH accounts for up to 10% of all strokes and is the most common cause of sudden death from a stroke.50 Approximately 80% of patients with nontraumatic SAH have ruptured saccular aneurysms.15 Other causes include arteriovenous malformations, cavernous angiomas, mycotic aneurysms, neoplasms, and blood dyscrasias. SAH may be caused secondarily by an intraparenchymal hematoma that dissects its way into the subarachnoid space. The risk for aneurysmal SAH increases with age; most cases occur between the ages of 40 and 60 years.51 In children and adolescents, aneurysms are uncommon, and when SAH occurs, it usually is secondary to an arteriovenous malformation. It is estimated that 2% of the general population harbor a berry aneurysm, and the risk of rupture may increase with aneurysmal size.52 Other risk factors associated with SAH include hypertension, smoking, excessive alcohol consumption, and sympathomimetic drugs.53 Increased systolic blood pressure values and long-term hypertension before aneurysm rupture seem to predict fatal SAH independently of aneurysm size or the patient’s age at the time of rupture; patient gender also does not influence mortality.54 A familial association of cerebral aneurysms with several diseases, including autosomal dominant polycystic kidney disease, coarctation of the aorta, Marfan syndrome, and Ehlers-Danlos syndrome type IV, has been described. Of all patients presenting to the ED with a primary complaint of headache, less than 1% have SAH. Many patients with SAH die before reaching the hospital; preadmission mortality rates range from 3 to 26%.15 Because of the significant morbidity and mortality associated with this condition and the high likelihood of clinical deterioration in patients who initially are misdiagnosed, SAH should be a primary consideration in the initial ED evaluation of all patients with nontraumatic headache. Accordingly, familiarity with its presentation is essential. A majority of patients with SAH present with a sudden, cataclysmic thunderclap headache, which often is described as “the worst headache of [their] life.” The onset of headache may be associated with exertion, the Valsalva maneuver, or sexual intercourse in up to 20% of patients.15 The headache of SAH classically peaks in intensity within seconds to minutes. Headaches that take longer to peak in intensity are less likely to be SAH.55 Associated signs and symptoms include nausea and vomiting in approximately 75% of patients, neck stiffness in 25%, and seizures in 17%.51 Physical findings depend on the extent of the SAH. Meningismus is present in more than 50% of patients, and up to 20% have focal neurologic abnormalities.56 Funduscopic examination may reveal retinal or subhyaloid hemorrhages, and patients also may have an isolated third or sixth nerve palsy. Oculomotor (third) nerve compression secondary to an expanding aneurysm leads to pupillary dilation. Approximately 50% of patients with a ruptured aneurysm are restless or have an altered level of consciousness. Although a majority do not have focal neurologic signs, such signs when present may indicate the site of the aneurysm.57 The patient’s prognosis is related to neurologic status at hospital admission. The Hunt and Hess scale stratifies patients according to their clinical signs and symptoms at the time of presentation and is predictive of outcome (Table 103-2).58 Patients who present with a grade 1 or grade 2 hemorrhage tend to have a good prognosis. Patients with grade 4 or grade 5 hemorrhage tend to do poorly, and these patients have an altered mental status, ranging from stupor to deep coma, together with focal neurologic signs and symptoms. Patients with grade 3 hemorrhage present with drowsiness or confusion and are at risk for rapid clinical deterioration. Table 103-2 Hunt and Hess Clinical Grading Scale for Cerebral Aneurysms and Subarachnoid Hemorrhage A non–contrast-enhanced head CT scan is the first-line test for the diagnosis of SAH and should be ordered emergently when this diagnosis is suspected (Fig. 103-1). For acute hemorrhage less than 24 hours old, the sensitivity of CT in identifying hemorrhage is greater than 90%; however, sensitivity decreases to approximately 50% by the end of the first week.59 Because the sensitivity of head CT is limited, particularly as the duration of headache increases, the cerebrospinal fluid (CSF) should be examined for blood or xanthochromia, the yellowish tinting of CSF by hemoglobin breakdown products, for SAH to be ruled out definitively. A common pitfall in ED diagnostic workups of headache is to overestimate the sensitivity of the CT scan for subarachnoid blood. Alternatively, an appropriate strategy may be to perform lumbar puncture without first performing a CT scan to decrease throughput time in carefully selected young patients who have completely normal physical examination findings, who are not immunocompromised, and in whom SAH is not strongly suspected.60 This strategy may be particularly appropriate for patients in whom the differential diagnosis includes other conditions requiring a CSF analysis, such as meningitis. An alternative strategy is to perform a non–contrast-enhanced head CT scan followed by CT angiography rather than the lumbar puncture; this is reasonably sensitive and may be appropriate for patients at lower risk of disease.59 Concerns of additional radiation exposure and contrast agent toxicity need to be weighed against the pain caused by a lumbar puncture. Finally, several published clinical decision rules can help risk stratify patients.16,61 Figure 103-1 Cerebral aneurysm. Shown is a computed tomography scan of an aneurysmal subarachnoid hemorrhage in a 55-year-old woman. Subarachnoid blood can be seen within the interpeduncular and ambient cisterns and the right sylvian fissure from a ruptured aneurysm at the junction of the right carotid artery and the posterior communicating artery. (From Brisman JL: Neurosurgery for cerebral aneurysm. Emedicine, updated Sep 23, 2010. Available at http://emedicine.medscape.com/article/252142-overview#a1.)
Headache Disorders*
Primary Headache Disorders
Principles of Disease
Clinical Features
Diagnostic Evaluation
Treatment
Prophylactic Therapy
Cluster Headache
Clinical Features
Differential Diagnosis
Treatment
Tension-type Headache
Clinical Features
Differential Diagnosis
Treatment
Secondary Headache Disorders
Principles of Disease
Clinical Features
GRADE
CONDITION
0
Unruptured aneurysm
1
Asymptomatic or minimal headache and slight nuchal rigidity
2
Moderate or severe headache, nuchal rigidity, no neurologic deficit other than cranial nerve palsy
3
Drowsiness, confusion, or mild focal deficit
4
Stupor, moderate to severe hemiparesis
5
Deep coma, decerebrate posturing, moribund appearance
Diagnostic Studies
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Headache Disorders
Only gold members can continue reading. Log In or Register to continue