and Joseph J. Stirparo1
(1)
Chief Academic Officer, Lehigh Valley Health Network, Associate Dean for Educational Affairs, USF Morsani College of Medicine – Lehigh Valley, 1247 S. Cedar Crest Blvd, Suite 202, Allentown, PA 18103, USA
Introduction
Psychological disorders and other cognitive impairments adversely affect outcomes in hospitalized elderly [1]. This has also been demonstrated in those with orthopedic injury [2–5]. Dependence in activities of daily living and moderate-to-severe cognitive impairment were independent risk factors for in-hospital mortality [6]. Despite these known associations, there continues to exist a dearth of information in the trauma literature. Conflicting results may be related to inaccuracies in the diagnoses of dementia and delirium [7]. In this chapter, we discuss the essentials of several neuropsychiatric disorders prevalent in the geriatric trauma patient.
Cognitive Disorders/Dementia
The DSM-IV describes dementia as the development of multiple cognitive deficits that include memory impairment and at least one of the following:
Aphasia – Inability to understand or express speech
Apraxia – Inability to perform purposive actions
Agnosia – Inability to interpret sensory inputs
Disturbance in executive functioning
The cognitive deficits must be sufficiently severe to cause impairment in occupational or social functioning and represent a decline from a previous higher level of functioning. If the deficits occur only during the course of delirium, the diagnosis of dementia should not be made. Both may be diagnosed if the dementia is present at times when the delirium is not present [8].
Despite increased awareness, dementia is often moderate to severe by the time it is diagnosed. Overall, women exhibit dementia and cognitive impairment more often than males. Alzheimer’s disease (AD) is more common in women, and vascular dementia is more common in men.
Elderly with dementia are more likely to be admitted for fractured femurs, lower respiratory tract infections, urinary tract infections, and head injuries than those without dementia. Mean length of stay for admissions for patients with dementia was 16.4 days compared to 8.9 days for those without dementia. Additionally, elderly with dementia were more likely than those without to be readmitted within 3 months. Mortality rates and transfers to nursing home care were higher for those with dementia than without. Outcomes were more pronounced in younger patients with dementia (Table 9.1) [1].
Table 9.1
Comparison of common types of dementia
Alzheimer’s | Lewy body | Frontal lobe | Vascular | |
|---|---|---|---|---|
~% of cases | 50% | 20% | <10% | 20% |
Pathology | Neurofibrillary tangles | Lewy bodies in cortex and amygdala | Degeneration of frontal regions | Varied vascular |
Distinct features | Progressive memory loss | Parkinson-like symptoms; hallucinations; transient loss of consciousness | Behavioral and personality changes | Varied depending on location |
Potential medical treatments | Cholinesterase inhibitors and memantidine | No specific treatment; cholinesterase inhibitors for cognitive symptoms; Parkinson’s meds for motor symptoms | No specific treatment; Alzheimer’s meds may worsen symptoms | No specific treatment, prevent ongoing vascular events |
Types of Dementia
Dementia is characterized by an acquired and persistent deficit in cognitive domains that interferes with daily functioning. There are several different types of dementia. Alzheimer’s disease is the most common type of dementia representing 50–70 % of cases. Vascular dementia can have a number of different etiologies and is second most common. Frontal lobe dementia and Lewy body dementia round out the most common causes. It is estimated that one in three seniors will die with a diagnosis of dementia.
Alzheimer’s Disease
Alzheimer’s disease (AD) is progressive and irreversible and eventually leads to death [9]. At least 5.3 million people in the United States are affected. It is the sixth leading cause of death in adults. One in eight adults over age 65 and half of those over age 85 have the disease. Direct costs for patients with Alzheimer’s disease are $200 billion, of which Medicare and Medicaid pay $140 billion. By the year 2050, over $1.1 trillion are estimated to be spent on Alzheimer’s disease [10].
Neurofibrillary tangles and neuritic plaques are the characteristic lesions in brains with Alzheimer’s disease. Beta-amyloid protein is present in these lesions and may play a central role in the disease as a deficit of acetylcholine and cholinergic areas [11, 12]. A history of head trauma as well as vascular disease increases the risk of Alzheimer’s disease. Clinical features include progressive memory loss, impairment of language, visuospatial ability, and executive function. As in most dementias, behavioral and psychotic symptoms may occur.
Vascular Dementia
The term vascular dementia applies to many causes of dementia, including multi-infarct dementia and small vessel disease. About one-fifth of all cases of dementia are vascular. Many of the same things that put one at risk for cardiovascular disease also put one at risk for vascular dementia. These include diabetes, hypercholesterolemia, hyperhomocysteinemia, hypertension, cigarette smoking, and physical inactivity. The effects of these risk factors may vary depending on the type of vascular dementia. Some types are related to specific gene mutations but are rare.
The presenting symptoms and signs will depend on the location of the lesions. Left hemisphere lesions usually cause language problems, and right hemisphere lesions generally cause visuospatial problems. The course may be stepwise, either with abrupt declines or more insidious. Memory or mood complaints are common in both vascular and Alzheimer’s disease. Recognition memory is often preserved in vascular dementia, not in Alzheimer’s. Isolated psychotic symptoms, apathy, and higher cortical disturbance with intact memory may also be seen in vascular dementia.
Lewy Body Dementia
Lewy body dementia is characterized by Parkinson-like symptoms, recurrent visual hallucinations, neuroleptic sensitivity, fluctuating cognition, falls or syncope, and a transient loss of consciousness. Lewy body dementia may account for up to 20 % of dementia in the United States, affecting up to 1.3 million people. Only 30–50 % of cases are accurately diagnosed [13].
Hallmark lesions are protein deposits known as Lewy bodies. These deposits are located in the cortex and amygdala in Lewy body dementia, as opposed to Parkinson’s, where the deposits are in the brain stem and substantia nigra.
Both the cholinergic and dopaminergic systems are severely disrupted. Other causes for cognitive decline in the setting of parkinsonism should be excluded with testing.
Frontal Lobe Dementias
Frontotemporal dementias are a heterogeneous group of disorders that involve degeneration of different regions of the frontal and temporal lobes. Clinically, behavioral and personality changes may predominate over cognitive deficits. These include a loss of personal or social awareness, a lack of insight, inappropriate and stereotyped behaviors, aggression, distraction, a loss of inhibitions, apathy, or extroverted behavior. Some cases involve language and aphasia as primary characteristics. The majority of cases of this group of disorders occur in those under 65 years of age.
As this is a group of disorders, the pathophysiology is not well understood.
The cognitive, neuropsychiatric, and behavioral symptoms depend on the regions of the brain involved. Though beginning as a regional process, as the disease progresses, the atrophy and pathology become more generalized.
Cognitive Screening Tests
One must first rule out potentially reversible cognitive deficits due to underlying disorders. Laboratory and imaging tests should be done as indicated. Examples of acute reversible disorders resulting in cognitive deficits include hypercapnia, hypoxemia, hypoglycemia, electrolyte disturbances, sepsis, and traumatic brain injury. Disorders that can mimic dementia include depression, delirium, anticholinergic medications, and toxic metabolic encephalopathy.
There are several standardized screening tests for cognitive impairment. One of the oldest and most well-known is the Mini-Mental State Examination (MMSE) [14]. It is a 30-point questionnaire which takes about 10 min to complete. It can be used to evaluate a patient at a point in time and then repeated to check response to treatment. Categories assessed are orientation to time and place, registration, attention and calculation, recall, language, repetition, and complex commands. A score of 25 or greater is normal. Scores of 21–24, 10–20, and ≤9 indicate mild, moderate, and severe cognitive impairment, respectively. Adjustments may need to be made to the raw score for educational level and age.
The Mini-Cog is a quick and simple method of screening for cognitive dysfunction [15]. It takes about 3–5 min to complete. It consists of three-item recall and clock drawing. Clock drawing was used to clarify scores when memory was intermediate. Recall of none of three items signified dementia. Recall of one or two items signified dementia when accompanied by an abnormal clock drawing test. Recall of all three items was considered normal. When compared to the MMSE, the Mini-Cog had better sensitivity at 99 % and correctly classified the greatest percentage (96 %) of subjects. Its diagnostic value was not influenced by education or language [15].
The Montreal Cognitive Assessment (MoCA) is a one-page 30-point test that takes about 10 min to complete [16]. The domains assessed are short-term memory recall, visuospatial abilities, executive functions attention, concentration and working memory, language, and orientation to time and place. MoCA may be better for mild cognitive impairment and early dementias as well as other neurological disorders that affect younger patients such as Huntington’s and Parkinson’s diseases [17–21].
The Saint Louis University Mental Status (SLUMS) Examination is an alternative to the MMSE. In a large study comparing the two tests, SLUMS was able to better detect mild neurocognitive disorders [22]. Sensitivity and specificity of the two tests are comparable. The test takes about 10 min to administer and includes a clock drawing test.
Overview of Treatments
Treatment of dementias is complex and varies both on type of dementia and severity of symptoms. Due to this complexity, treatment is best when practiced with a multidisciplinary approach including geriatricians, pharmacists, and neurologists. A brief overview of potential treatments is provided below. Practitioners should discuss treatment plans with a multidisciplinary team.
Alzheimer’s disease had several FDA-approved drug therapies. The cholinesterase inhibitors and memantine are used to address cognitive symptoms. Patients may survive as long as 20 years with Alzheimer’s disease, but many patients succumb in the early or middle stages of the disease.
Lewy body dementia has no FDA-approved treatment. As there are cholinergic losses and relationship with Alzheimer’s disease, cholinesterase inhibitors were found to have a role and have become standard treatment for cognitive symptoms [23]. Low doses of Parkinson’s medications, i.e., levodopa, may help motor symptoms, but caution must be used as higher doses can worsen neuropsychiatric symptoms. Average duration of illness is 5–7 years but with much variability.
Frontal lobe dementias have no specific treatments or cures. Not all cases have the same underlying pathology. Cholinesterase inhibitors and memantine may worsen behavioral and psychological symptoms. Long-term care is necessary as the average duration between onset of illness and death is 7 years.
There are no specific treatments for vascular dementia. Control of vascular risk factors is primary. Behavioral and psychological features are treated as necessary.
Behavioral and psychological symptoms including depression occur in the majority of patients with dementia. Psychological symptoms include delusions, hallucinations, paranoia, anxiety, and apathy. Behavioral symptoms include wandering, aggression, hostility, insomnia, inappropriate eating, and abnormal sexual behaviors [24].
Non-pharmacological strategies should be employed in all acute care facilities and are the first-line therapies for behavioral and psychological symptoms in dementia. These consist of environmental and behavioral interventions such as regularly scheduled routines for meals, sleep, and bathing. Reorientation with a clearly visible clock and calendar is indicated. Caregivers should use clear instructions and make frequent eye contact with patients. Sensory impairments, such as vision and hearing loss, should be minimized. These will be addressed in more depth in the section on delirium.
Pharmacological interventions are variable depending on the type of dementia. When medication is necessary, neuropsychiatric symptoms in many dementias have been treated with antipsychotics. Atypical antipsychotics may be better than typical [25]. Quetiapine has been used for psychosis in parkinsonian syndromes [26]. There are concerns regarding the use of these agents. First-generation antipsychotics produce more extrapyramidal symptoms. The second-generation antipsychotics have had a “black box” warning label added by the US Food and Drug Administration for a small but statistically significant increase in cerebrovascular events and death. The older antipsychotics also carry an increased risk of death [27]. A recent cohort study looking at over 75,000 elderly nursing home patients using antipsychotics found that haloperidol had a higher risk of dying when compared with risperidone [28]. Quetiapine users also had a decreased risk of mortality. A dose-response relation was noted with all drugs but quetiapine.
Patients with dementia may have paradoxical agitation when given benzodiazepines. Tricyclic antidepressants may have unwanted anticholinergic effects. Mood stabilizers, especially selective serotonin reuptake inhibitors (SSRIs), may help neuropsychiatric features of frontotemporal dementias.
Cholinesterase inhibitors have been used for neuropsychiatric symptom treatment of Alzheimer’s disease and vascular dementias since cholinergic deficiency also appears to be involved in their development [29]. However, when used in frontotemporal dementias, they may worsen these symptoms.
Neuropsychiatric symptoms in Lewy body dementias can be challenging to treat medically. Older antipsychotic drugs may cause worsening of symptoms and neuroleptic malignant syndrome in Lewy body dementias. As mentioned previously, newer antipsychotics seem to be more beneficial. Benzodiazepines, anticholinergics, and some antidepressants may cause sedation, motor impairment, or confusion. Medications for parkinsonian symptoms may also worsen confusion, delusions, and hallucinations in higher doses.
In summary, the first-line therapies for neuropsychiatric and behavioral symptoms should be non-pharmacological, and medications should be used judiciously and with caution. As the principle of geriatric pharmacological intervention states, “start low, go slow but go.” Limit medications to necessity, again in a multidisciplinary format.
Delirium
Definition and Epidemiology
Delirium is a transient, reversible syndrome of impairment of consciousness, attention, and perception in the setting of a medical condition that is acute and fluctuating. The roots of the word are Latin with the term coined by Celsus and included in his work De Medicina. Deconstructing the work from its Latin root, “de” is for “away from” and “lira” is for “furrow in a field.” Literally translated it means “going off track [30].”
Delirium occurs in up to 60 % of hospitalized frail elderly patients [31]. One study found that 89 % of survivors of stupor or coma progressed to delirium [32]. Similarly, in the general surgical population, the incidence of delirium is 37–46 %, and postoperative delirium has been described to occur in 10–60 % of patients [33, 34]. The range in incidence of postoperative delirium depends on the type of surgery and the population studied. For example, the incidence of delirium was found to be 65 % after femoral neck fracture repair [35–37]. Approximately seven out of ten surgical intensive care and trauma intensive care patients experience delirium [38].
There exist three subtypes of delirium: hyperactive, hypoactive, and mixed. Hyperactive delirium occurs when the patient exhibits three or more of the following: hypervigilance, restlessness, fast and/or loud speech, anger, irritability, combativeness, impatience, uncooperativeness, laughing, swearing, singing, euphoria, easy startling, distractibility, nightmares, persistent thoughts, and wandering. For hypoactive, the most difficult to diagnose and identify, the patient must exhibit four or more of the following: unawareness, lethargy, decreased alertness, decreased motor activity, staring, sparse and/or slow speech, and apathy [39]. Mixed, which is the most common subtype, contains features of both.
Delirium has been shown to increase mortality when other factors are controlled for. Studies have shown that the mortality rate for patients with delirium was significantly higher with regard to interval or patient population studied. Mortality rates for patients with delirium compared to those without range from 8 % vs 1 % in hospitalized inpatients, 34 % vs 15 % for 6-month mortality after ICU stay, and 11 % vs 3 % for 90-day mortality in med-surg patients [40–42].
The costs of delirium can be staggering, ranging from $38 billion to $152 billion per year in a study of healthcare costs [43]. Though patients with delirium survived fewer days than those without, they had significantly higher adjusted costs, over 2.5 times the costs of patients without delirium. Costs attributable to delirium were $16,303 to $64,421 per patient.
Causes and Risk Factors
There are many causes and risk factors for delirium. Temporal relationship to clinical events is an important clue to cause. For example, exposure to midazolam is an independent and potentially modifiable risk factor for the development of delirium [38]. Delirium arising after administration of midazolam would point to the drug as the cause.
The cause may also be determined by the clinical situation or condition. Potentially life-threatening conditions which cause delirium can be remembered by the mnemonic WHHHHIMPS. They are Wernicke’s disease, hypoxia, hypoglycemia, hypertensive encephalopathy, hyperthermia or hypothermia, intracerebral hemorrhage, meningitis/encephalitis, poisoning, either exogenous or iatrogenic, and status epilepticus.
Other risk factors can be divided into potentially modifiable and non-modifiable. Non-modifiable risk factors include dementia or history of cognitive impairment, age over 65, chronic renal or hepatic disease, multiple comorbidities, and a history of delirium, stroke, neurological disease, falls, or gait disorder. Potentially modifiable risk factors include surgical pain, concurrent illness, acute neurological diseases, medications, immobilization including by catheters or restraints, sensory impairment of hearing or vision, metabolic derangements, environment, emotional distress, and sustained sleep deprivation. The categories of causes can be remembered by the mnemonic I WATCH DEATH. The table explains the mnemonic (Table 9.2). There exists suggestion that length of operative procedure (greater than 3 h), utilization of general anesthesia, intraoperative hypercapnia, and hypotension may increase incidence of delirium [85].
Table 9.2
Causes of delirium
Categories | Examples |
|---|---|
Infectious | Encephalitis, meningitis, pneumonia, urinary tract infection |
Withdrawal | Alcohol, sedative-hypnotics |
Acute metabolic | Acidosis, alkalosis, electrolyte disturbances, hepatic or renal failure |
Trauma | Heat stroke, burns, surgery |
CNS pathology | Hemorrhage, seizures, stroke, tumors, vasculitis, hydrocephalus |
Hypoxia | Hypoxia from cardiac or pulmonary cause, anemia, carbon monoxide poisoning, hypotension |
Deficiencies | Vitamin B12, niacin, thiamine |
Endocrinopathies | Disorders of glucose, cortisol, thyroid and parathyroids |
Acute vascular | Hypertensive encephalopathy, shock |
Toxins or drugs | Medications, toxins |
Heavy metals | Lead, manganese, mercury |
Dementia as a risk factor was discussed in the previous section. Two studies help accentuate its importance. Wahlund and Bjorlin in 1999 found that approximately 70 % of elderly patients admitted to a specialized delirium ward had either dementia or mild cognitive impairment [44]. In a study of total joint replacement patients, all demented patients postoperatively developed delirium, compared with 31.8 % in the non-demented patients [37].
Age is a major risk factor for delirium and deserves special mention. One study suggests this relationship is linear after age 65. In mechanically ventilated patients, the probability of developing delirium increased by 2 % for each year over age 65 [45]. Hypoxia is a well-known risk factor for delirium. Not only poor oxygenation but also poor oxygen delivery, i.e., anemia, can contribute to delirium [46, 47]. Hypoxia can come from many causes, even obstructive sleep apnea [48].
Many medications have the capacity to cause delirium. This is especially true of those with psychoactive effects and those with anticholinergic effects. There appears to be a direct relationship between a drug’s anticholinergic properties and the development of delirium [49–51]. A study by Han et al. found that exposure to anticholinergic agents was an independent risk factor for the development of delirium and an increase in symptom severity [52].
Dr. Mark Beers created the first Beers Criteria list in 1991 with a consensus panel of experts. It has been updated several times, most recently in 2015 as the American Geriatrics Society Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. The list identifies medications or classes of medication that are potentially inappropriate in all older adults, and medications should be used with caution [53]. In older adults with certain diseases and syndromes, the drugs listed can exacerbate symptoms. All practitioners caring for the elderly must be aware of this list and utilize it to the benefit of their patients. The most recent update of the criteria will be used as an educational tool and a quality measure.
Diagnosis and Screening
Delirium is unrecognized in many cases. The literature estimates that from 65–84 % of cases go undiagnosed. Delirium can mimic many other mental illnesses. A cornerstone of diagnosis is identification of the underlying causes and correcting modifiable ones.
There are a host of objective diagnostic and screening tests for delirium (Table 9.3). In December 2008, the Canadian Agency for Drugs and Technologies in Health published a review of evidence-based guidelines on diagnostic tests for delirium. They reviewed the 2006 Canadian Coalition for Seniors’ Mental Health (CCSMH) published evidence-based guidelines for the assessment and treatment of delirium, the 2006 British Geriatrics Society and the Royal College of Physicians’ evidence-based delirium guidelines, and the 2007 delirium guidelines for general hospitals by Swiss and French physicians [57–60]. They concluded that early assessment for delirium is needed in hospitalized elderly patients. This early detection of risk factors may prevent delirium and its complications. Physicians and nurses must be educated to recognize delirium in the use of validated screening and diagnostic tools. They suggested that the Confusion Assessment Method be used for screening and diagnosis, and the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) criteria be used to confirm the diagnosis.
Table 9.3
Objective tests to diagnose delirium
Cognitive Test for Delirium (CTD) |
Confusion Assessment Method (CAM and Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) |
Confusional State Evaluation (CSE) |
Delirium Assessment Scale (DAS) |
Delirium Detection Score (DDS) |
Delirium Index (DI) |
Delirium Rating Scale and Delirium Rating Scale-revised-98 (DRS) |
Delirium Severity Scale (DSS) |
Delirium Symptom Interview (DSI) |
Memorial Delirium Assessment Scale (MDAS) |
Short Portable Mental Status Questionnaire (SPMSQ) |
In 2010, the National Clinical Guideline Centre of Britain published its clinical guideline titled “Delirium: Diagnosis, Prevention and Management.” As to screening and diagnosis, they recommended screening for behavioral changes at presentation and daily during hospital admission. If indicators of delirium are identified, they recommend that a healthcare professional who is trained and competent in the diagnosis of delirium carry out a clinical assessment based on the DSM-IV criteria or Confusion Assessment Method short version (short CAM) to confirm the diagnosis. The CAM-ICU should be used when patients are intubated in the ICU or recovery room postoperatively [61].
The Confusion Assessment Method was unveiled in 1990 and the Confusion Assessment Method-ICU in 2001 [62, 63]. Both have a sensitivity of 94–100 %, a specificity of 89–95 %, and high inter-rater reliability [64]. The CAM has a long and a short version. The long version is comprehensive and screens for nine clinical features. The short version focuses on the four features that have the greatest discriminatory ability to detect delirium from other cognitive disorders. There is also a version designed for use in the Emergency Department, the brief CAM (bCAM). Another rapid rule-out tool is the Delirium Triage Screen (DTS). Validated in Emergency Departments, it is a 20 s tool that is 98 % sensitive and 55 % specific for delirium as diagnosed by a psychiatrist assessment.
The CAM-ICU addresses the same four areas as the short version CAM. It was developed by Ely et al. at Vanderbilt. It takes less than 2 min to complete and can be given to intubated patients. The process is shown in Fig. 9.1.
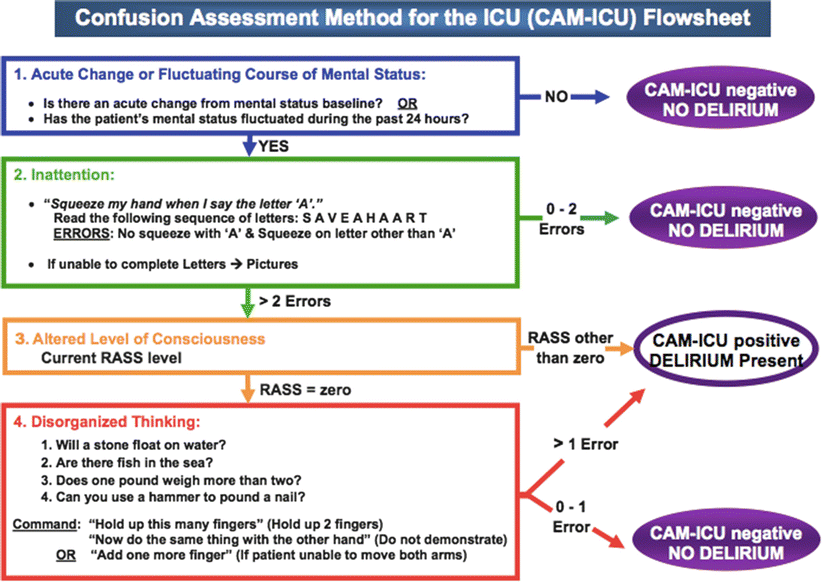

Fig. 9.1
CAM ICU flowsheet
Prevention and Treatment
In the case of delirium, prevention is the best medicine. The National Clinical Guideline Centre of Britain addressed prevention in its clinical guideline titled “Delirium: Diagnosis, Prevention and Management [61].” The American Geriatrics Society published an abstracted clinical practice guideline for postoperative delirium in older adults [84]. The following are a summary of the findings of these reviews supplemented by additional sources as indicated.
Delirium can be difficult to recognize and treat. People at risk for delirium should be under the care of an interdisciplinary team that is “trained and competent in delirium prevention.” It would be best if the team were familiar to the patient at risk. Patients at risk should remain with the same caregivers and not change units unless necessary. A tailored multicomponent program should be administered which includes environmental change and non-pharmacological interventions. Rather than recommend a particular program, they chose to focus on the elements that should be addressed. The early assessment of risk is key to this process.
Changes to ensure good sleep patterns include changes to the method of carrying out clinical care. Nursing and medical procedures should be avoided during sleep periods. This includes administration of medication as possible. Noise should be reduced to a minimum during these times as well.
Attention must be paid to medications administered. Utilization of a tool such as the Beers Criteria list mentioned earlier will help identify medications placing the patient at risk and suggest substitutes. Including a pharmacist on the multidisciplinary team can help.
Patients requiring operative intervention should have special attention to perioperative prevention of delirium. Electroencephalographic monitors of anesthetic depth for general anesthesia may decrease delirium incidence. Regional anesthetic should be considered when possible. Postoperative pain control should be optimized. Attention should be paid to non-opioid pain medications whenever possible [85].
Closely assessing for and correcting hypoxia is essential. The same is true for infection. Occult infection can present as delirium. Infection control procedures including reducing use of catheters remain important.
Any sensory impairment that can be improved or resolved should be addressed. Removing impacted earwax and ensuring the availability and use of working hearing and visual aids are among the interventions that can reduce risk.
Lack of or impaired mobility is another risk factor that can be addressed. Early postoperative mobilization should be encouraged whenever possible. Assistive devices should be readily available. Even those who cannot walk should be encouraged to perform active range-of-motion exercises. Physical therapy or rehabilitation medicine can help as part of the multidisciplinary team.
Dehydration and constipation can be detrimental risk factors. Appropriate hydration can be maintained by oral, subcutaneous, or intravenous fluids depending on the status of the patient. Consult as necessary for patients with comorbidities such as heart or renal failure. A bowel regimen should be used with a stepwise approach for prophylaxis and treatment. Nutrition must be maintained. Dentures should be properly fitting and available when needed.
Pain must be assessed by whatever means appropriate. Pain management should be undertaken if not already in place. Pain medication should be reviewed if already being administered.
Environmental and practice changes can address cognitive impairment and/or disorientation. Steps include appropriate lighting, clear signage, and easily visible clocks and calendars. As mentioned earlier, clear communication with eye contact should be the norm when dealing with these patients. Frequent reorientation and reassurance can help. Regular visits from family and friends and activities to stimulate cognition, such as reminiscing, have a positive impact as well.
When patients become agitated or a danger to themselves or others, verbal and nonverbal techniques should be used to de-escalate the situation. When these techniques fail, pharmacological interventions should be considered for short-term use. Haloperidol or olanzapine is recommended in the NICE Guideline. Again, per the geriatric medication mantra, “start low, go slow but go.” Cautiously titrate to symptoms. Particular caution must be used with antipsychotics for those with Parkinson’s type diseases or Lewy body dementia if they are to be used at all.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





