The CFS was highly correlated (r = 0.80) with the full FI and much easier to apply. Each one-category increment of the scale significantly increased the medium-term risk of death (21.2 % within about 70 months, 95 % confidence interval CI 12.5–30.6 %) and entry into an institution (23.9, 95 % CI 18.8–41.2) in multivariable models adjusted for age and sex [38].
Frailty has been predictive of increased mortality, postoperative complications, and discharge to a nursing facility in elderly injured and surgical patients [39, 40]. In a meta-analysis by Kojima et al., prevalence of frailty in nursing home residents is 40–50 % which predicts poor outcome for many of these patients [41]. This data corresponds with a recent large-scale study by Newman et al. evaluating survival and functional outcomes after hip fracture among nursing home residents that demonstrated a 36.2 % mortality in 180 days and functional decline in all activities of daily living domains assessed [42].
The British Geriatrics Society recommends a comprehensive geriatric assessment (CGA) for all older people identified with frailty. This will identify medical illnesses, allowing treatment optimization; apply evidence-based medication review checklists, such as the STOPP/START criteria which are discussed later in the chapter; establish dialogue with older people and their caregivers to assess the impact of illness or injury; and create an individualized care plan that is congruent with patient’s goals and beliefs.
Those who are defined as vulnerable or mildly frail (groups 4 and 5 on CFS) and/or who met some but not all criteria for frailty as assessed by Fried’s phenotype may benefit the most from the input of a multidisciplinary team. These elders have potentially reversible issues that, if addressed early, will allow for longer functional independence. Very frail injured elders (groups 7, 8, and 9 on CFS) may benefit from referral to palliative care specialists who can assist the trauma team in discussing goals of care with patient and family, weighing the risks and benefits of any proposed treatment with quality of life after the injury [43, 44].
Falls
Fall-related injuries are among the leading causes of functional impairment, loss of independence, and death in older adults. Falls are common in community-dwelling older people with an incidence of about 30 % annually for those over the age of 65 [45] and increasing up to 40 % for those over the age of 80 [46]. Falls are even more common in nursing the home residents, with between 50 and 75 % of residents falling every year [47].
Falls rarely have a single cause: most are multifactorial [48], with the risk of falling increasing in a linear manner with the number of risk factors involved [46]. The most common risk factors for falls are history of previous falls, gait and balance impairment, and medications [49]. In 1 year, 9 % of community-dwelling elders had more than one fall [50], and nearly 30 % of patients seen in the ED [51] and 40 % of admitted trauma patients [21] were recidivist, having had more than one fall. Medication use, especially the combination of polypharmacy and medications affecting central nervous system, has been shown to be closely associated with falls [52]. Cognitive impairment, especially deficits in executive function, has been shown to increase the risk of both falls and injurious falls [48, 53].
The fact that falls are a widespread and recurrent problem, with significant morbidity and mortality, and have identifiable risk factors makes falls (from a public health perspective) akin to an epidemic, which warrants preventive measures [54, 55]. Randomized controlled trials have investigated both single and multiple interventions. A meta-analysis of multiple trials showed multifactorial interventions to be more effective in reducing the risk of falls, although the effects were moderate. The most effective interventions were those aimed at improving strength and balance, such as exercises, especially Tai Chi, medication adjustment, and home safety modification [56, 57, 58]. A meta-analysis of fall prevention programs in nursing homes found that these programs produced a smaller number of falls, and fewer recurrent fallers, but had no effect on the overall number of those who fell [59]. A meta-analysis from 2009 showed a 9.5 % absolute risk reduction in falls among community-dwelling and institutionalized adults given supplementation with 700 to 1000 i.u. of vitamin D daily [60].
Dissemination of evidence about fall prevention, coupled with interventions to change clinical practice, undertaken as part of Connecticut Collaboration for Fall Prevention (CCFP) initiative, showed a lower rate of hospitalizations for injurious falls [61] and for traumatic brain injury [62] in the treatment regions versus usual care regions.
And yet, despite the evidence that falls, and especially injurious falls can be prevented with a multifactorial prevention program and despite the fact that even minor falls may affect function in the frail elderly patients [63], many elders presenting to the ED or even hospitalized following a fall receive no education on these interventions. Most are not seen by a geriatrician or receive comprehensive geriatric assessment or any significant education on prevention strategies appropriate for them [64].
An excellent resource was compiled by researchers at the Centers for Disease Control and Prevention’s (CDC) Injury Center who created an algorithm based on the American and British Geriatric Societies’ Clinical Practice Guideline for falls screening and risk assessment. They used the mnemonic Stopping Elderly Accidents, Death, and Injury (STEADI). The site also includes basic information about falls, case studies, conversation starters, and description of standardized gait and balance assessment tests: the Timed Up and Go (TUG) Test, strength (the 30-Second Chair Stand Test), and balance (the Four-Stage Balance Test). This information is readily available at [65] http://www.cdc.gov/homeandrecreationalsafety/Falls/steadi/about.)
Polypharmacy
There is no standard definition of polypharmacy; multiple studies have defined this differently from “four or more” to “more than nine medications” daily. However, there is no doubt that it is common in the older adults. Surveys of community-dwelling elderly showed that on average two to nine prescription medications are taken daily by American seniors [66], while other studies, conducted in the United States and other countries, found out that 51 % of elderly people took more than six prescription medications a day, to say nothing of nonprescription medications [67]. This burden is especially heavy in the case of frail patients, who frequently have multiple comorbidities [68].
While there is a nonlinear association between the number of medications used and the frequency of adverse drug events (ADE) with the risk of ADE increasing fourfold for those taking eight or more medications [69], certain classes of medications have significantly higher risk.
In a large study by Budnitz et al. conducted between 2007–2009, there were nearly 100,000 emergency hospitalizations for adverse drug event annually for those over 65 years of age. The main drugs or medication classes implicated were warfarin and oral antiplatelet agents, which were associated with bleeding, and insulins and oral hypoglycemics, which were associated with severe hypoglycemia [70]. In this study, only 3.6 % of emergency department visits for ADE were associated with potentially inappropriate medication (PIM) use.
The Beers Criteria for potentially inappropriate medication (PIM) use in older adults, or “Beers list,” was created by Dr. Mark Beers and colleagues in 1991 and has undergone several revisions. The most recent was in 2015 by the American Geriatrics Society based on evidence-based guidelines and expert opinion of a 13-member panel. The recommendations were rated for the quality of the evidence supporting the panel’s recommendations and the strength of their recommendations. It comprises a list of medications to be avoided in older adults in general and in those with certain diseases or syndrome. There are additional lists of medications to be prescribed with caution and a table of medications with strong anticholinergic properties. The latest revision added list of drugs to be used with caution in patients with impaired kidney function and common drug-drug interactions [71].
The causative link between trauma and subdural hematoma for a patient on warfarin is generally obvious and seldom overlooked. The link between a fall or change in mental status and a PIM or polypharmacy may be less clear-cut, but there are numerous studies, implicating polypharmacy and/or the use of certain classes of medications, in both falls and delirium.
To illustrate, in the study by Richardson et al. [72], taking five or more medications, one of which was an antidepressant, was associated with a greater risk for falling, a greater number of falls, and a higher risk of injurious falls, but antidepressant use without polypharmacy and polypharmacy without antidepressants was not. The use of benzodiazepines was associated with injurious falls when coupled with polypharmacy (aRR 1.40, 95 % CI 1.04–1.87) but was also associated with a greater number of falls independent of polypharmacy (aIRR 1.32, 95 % CI 1.05–1.65). The risk of falling in an acute care hospital was increased more than threefold in those on tricyclic antidepressants and almost twofold on those on diuretics and narcotics [73]. The risk of falls in community-dwelling elders was found to be one and a half times greater in those who took one CNS active drug and 2.3 times greater in those who took more than one CNS active drug [74].
Polypharmacy and the use of psychoactive medications (benzodiazepines, anticholinergic, antihistamines, and antidepressants) have been identified as strong risk factors for postoperative delirium [75].
Thorough evaluation of elders presenting to the ED with nonspecific complaints, such as generalized weakness or dizziness, revealed 10 % increase in the probability of ADE with each additional drug, and this link was not recognized by the ED physician in 40 % of cases. The most commonly missed drug-related diagnoses were electrolyte abnormalities, such as hyponatremia [76]; another study recently linked hyponatremia with an increased risk of hip fracture in older persons [77].
Hospital admission after injury usually necessitates adding medications to address pain, nausea, deep venous thrombosis prophylaxis, and other possible complications of acute injury, predisposing injured elderly patients to polypharmacy and potential side effects, such as falls, delirium, constipation, and anorexia. The best way to approach polypharmacy in injured elderly patients is to review the medication list for medications that may be nonessential in the context of severe injury. Medications that may cause withdrawal, if discontinued abruptly (such as benzodiazepines), should be continued but consideration given to reducing the dose, as recommended by the jointly created American College of Surgeons National Surgical Quality Improvement Program and American Geriatrics Society ACS NSQIP ®/AGS Best Practices Guidelines regarding Optimal Preoperative Assessment of the Geriatric Surgical Patient [78]. Principles of appropriate prescribing should be used when adding new drugs, such as Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP) and Screening Tool to Alert Doctors to Right Treatment (START) criteria [79] and Beers criteria.
Pain Management
There is limited availability of evidence-based recommendations regarding the use of pain medications in older adults, because, although they are the fastest-growing segment of the population, they are largely underrepresented in clinical trials, despite their disproportionally high use of prescription drugs [80].
Elderly patients constitute a heterogeneous group and so predicting the optimal dose of medications, providing effective pain relief, while minimizing the complications such as delirium, nausea, and vomiting is difficult. The best approach is to start with low doses, 25–50 % of the usual starting dose, followed by careful upward titration until the goal of pain relief is achieved [81].
We will try to address some issues that deserve further elucidation when considering pain management in injured elders.
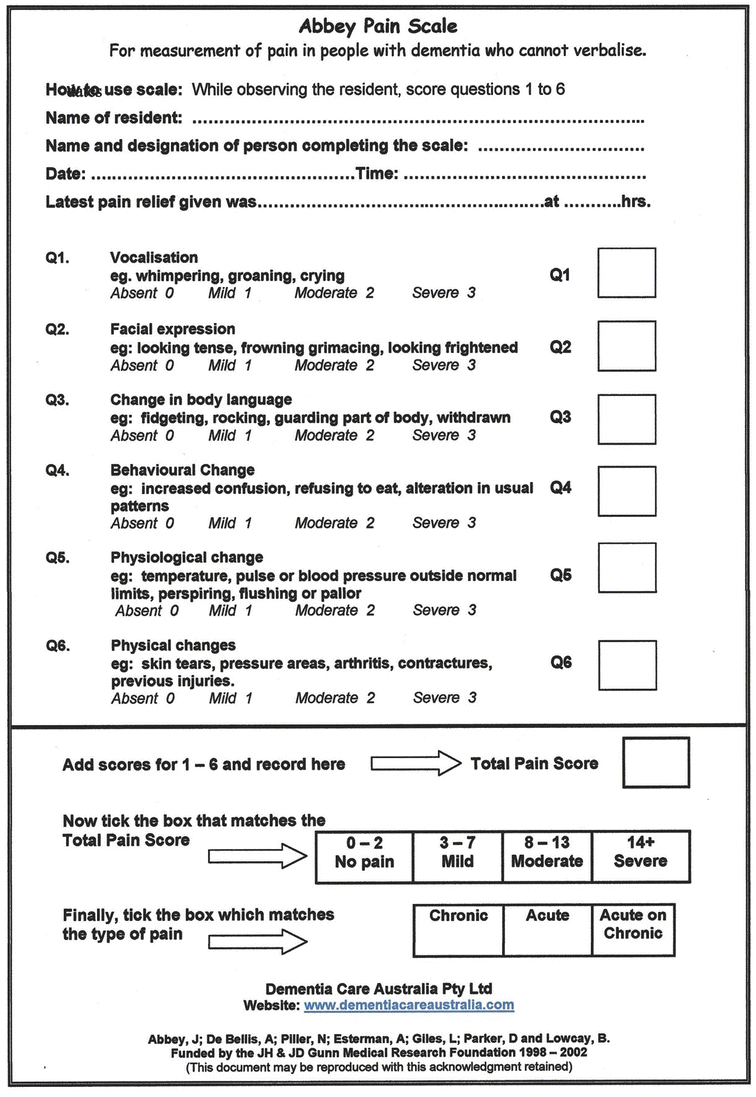
Pain is frequently underdiagnosed in older adults, especially those with cognitive impairment. Accurately assessing for pain presents the first challenge. Cognitively intact older adults and those with mild to moderate dementia are able to respond to simple questions and standard screening tools, rating their pain on traditionally used 1–10 scale. Patients with advanced dementia and nonverbal patients might be able respond to simple “yes” or “no” questions. Observation of facial expression or body language can give another clue and may be the only marker for nonverbal or intubated patients. Grimacing, frowning, or rapid blinking may be indicative of pain, as is guarding, rocking, or resistance to care. Caregivers, familiar with the patient, may observe change in behavior, usual pattern, or mental status. In a cognitively impaired patient, new onset of agitated or aggressive behavior may be caused by pain that the patient is unable to convey verbally and could be indicative of occult fracture. Although not validated to be used for trauma patient in randomized controlled trials, there are several tools that help to assess pain and its severity in cognitively impaired patients, such as the Abbey Pain Scale [82] and PAINAD scale [83]. The Abbey Scale assesses vocalization and behavioral and physiological changes, while the PAINAD scores behavioral changes only. When potential causes for pain, like a recent injury, are obvious, an empiric analgesic trial should be considered even if the patient is not complaining of pain.

Pain Assessment in Advanced Dementia Scale (PAINAD)
Instructions
Observe the patient for 5 min before scoring his or her behaviors. Score the behaviors according to the following chart. Definitions of each item are provided on the following page. The patient can be observed under different conditions (e.g., at rest, during a pleasant activity, during caregiving, after the administration of pain medication).
Pain Assessment in Advanced Dementia Scale (PAINAD)
Behavior | 0 | 1 | 2 | Score |
|---|---|---|---|---|
Breathing Independent of vocalization | 1. Normal | Occasional labored breathing Short period of hyperventilation | Noisy labored breathing Long period of hyperventilation Cheyne-Stokes respirations | |
Negative vocalization | None | Occasional moan or groan Low-level speech with a negative or disapproving quality | Repeated troubled calling out Loud moaning or groaning Crying | |
Facial expression | Smiling or inexpressive | Sad Frightened Frown | Facial grimacing | |
Body language | Relaxed | Tense Distressed pacing Fidgeting | Rigid Fists clenched Knees pulled up Pulling or pushing away Striking out | |
Consolability | No need to console | Distracted or reassured by voice or touch | Unable to console, distract, or reassure | |
Total score | ||||
Scoring
The total score ranges from 0 to 10 points. A possible interpretation of the scores is 1–3 = mild pain, 4–6 = moderate pain, and 7–10 = severe pain. These ranges are based on a standard 0–10 scale of pain but have not been substantiated in the literature for this tool.
The general principles of pain management are well established, with WHO’s 3-step ladder approach being the cornerstone. Non-opioids, such as acetaminophen, and oral or topical nonsteroidal anti-inflammatory drugs (NSAIDs) are step 1, and they remain first-line therapies for mild to moderate pain. Caution should be exercised when considering oral NSAIDs for patients with low creatinine clearance, cardiovascular diseases, or intravascular depletion state such as congestive heart failure. Step 2 for moderate pain includes mild opioids, or opioid-like drugs, such as tramadol, usually in combination with acetaminophen. Step 3 recommends the drugs for severe pain and includes narcotics such as morphine, fentanyl, and hydromorphone. Adjuvant medications and non-pharmacological treatment can be utilized at any step. Opioids should be started at 25–50 % of the recommended dose for adults [84].
Opioids have been implicated in falls and delirium, but this class of drugs is generally safe and provides effective analgesia for moderate to severe pain when initiated at low doses, and with preemptive strategies to minimize side effects. Untreated pain alone has been associated with postoperative delirium [85]. Age-associated changes in pharmacokinetics (absorption, distribution, metabolism, and elimination) and pharmacodynamics (increased or decreased sensitivity of the receptors to drugs) need to be recognized. Older patients are more sensitive to the effects of opioids. Changes in body composition cause a prolonged half-life of lipid-soluble drugs, such as fentanyl, necessitating longer intervals between the administrations of doses. Opioids are metabolized in the liver and excreted 90–95 % by the kidneys; therefore adjustments should be made for patients with hepatic and renal impairment [84].
Water-soluble opioids, such as morphine, achieve higher concentrations in plasma due to a relative decrease in total body water [86]. Morphine may cause severe respiratory depression in patients with renal impairment, due to accumulation of active metabolites. It may require a continuous infusion of naloxone rather than a bolus dose because the half-life of naloxone is much shorter than that of morphine-6-glucuronide, which can be up to 50 h in patient with renal failure [87]. Methadone is primarily excreted through the feces, but due to a prolonged half-life, it is not recommended in the elderly [88]. Buprenorphine is also excreted primarily via feces and considered a safe choice in patients with renal impairment [84]. Fentanyl is primarily metabolized in the liver; therefore it has a role in patients with advanced renal disease or acute renal failure [89].
All opioid medications have predictable side effects. Constipation is the most common due to binding to μ-opioid receptors located in the enteric nervous system which leads to increased non-propulsive contractions: patients never develop tolerance to this. All patients started on narcotics should be simultaneously started on stimulant laxatives, such as senna or bisacodyl, and the dose titrated up to achieve regular bowel movements. There are also newer (and more expensive) peripherally acting μ-opioid receptor antagonists such as naloxegol. These agents limit the effects of opioids on the gastrointestinal tract while preserving centrally mediated analgesia and have been shown effective for constipation without reducing opioid-mediated analgesia [90]. Stool softeners such as docusate are ineffective as monotherapy for opioid-induced constipation.
Other common opioid side effects are nausea and vomiting which can be treated with small frequent meals and either dopaminergic or serotonergic blocking agent, such as prochlorperazine or ondansetron. Opioids affect patients differently, and sometimes a change of type of opioid may achieve better pain control. It is important to follow opioid converter principles when changing between intravenous and oral route of administration and between different opioids.
Scheduled administration should be considered choice for patients with cognitive impairment who are not able to request medication appropriately. Scheduling medications 4 times a day, or while awake, rather than every 6 h, will avoid sleep interruption. The use of scheduled acetaminophen as a background pain medication may reduce the need for narcotics.
Most muscle relaxants (such as methocarbamol, cyclobenzaprine, metaxalone) are considered potentially inappropriate for older adults and should be avoided [71]. If used, treatment should be initiated at 25–50 % of the generally recommended dose. Long-acting benzodiazepines have a long half-life which could be further prolonged due to relative increase in adipose tissue and, therefore, larger volume of distribution. For example, the half-life of the active metabolite of diazepam can be up to 200 h leading to increased potential for the accumulation and side effects.
Non-pharmacological approaches, such as repositioning, use of ice or heat, and relaxation techniques, should be part of effective pain management strategies for all older adults.
Delirium
Delirium takes an especially high toll of surgical patients, especially those in the intensive care unit. As summarized in the first meeting of the American Delirium Society in 2011, the pathophysiology and neuropsychology of delirium are still poorly understood. While there is a correlation between delirium and such biomarkers, as S-100 beta and insulin-like growth factor-1 and inflammatory markers, no specific diagnostic studies are accurate to diagnose delirium, which remains a clinical diagnosis. While some studies have shown reduction in rates or duration of delirium, there are no proven medications to prevent or treat delirium [91]. As with many other geriatric symptoms, there is no single definitive causative pathway but rather many predisposing and precipitating risk factors, many contributing pathophysiologic pathways and multiple presentations, and therefore, a variety of preventative and treatment strategies [92].
Overall the risk of developing delirium depends on the relationship between the severity or the impact of a stressor and the quantity or severity of patient-associated risk factors [93]. The most common risk factors for delirium in hospitalized patients are age, underlying cognitive impairment, vision or hearing impairment, severe illness, and presence of infection [94]. It has been demonstrated in multiple studies that delirium in medical and surgical patients alike causes increased mortality, morbidity, prolonged length of stay and increases rates of functional decline and discharge to an institution [95, 96].
Prevention and management of delirium in a surgical patient, summarized in Clinical Practice Guidelines for Postoperative Delirium in Older Adults, are applicable to injured older adults [97]. Non-pharmacological interventions delivered by an interdisciplinary team are considered the approach of choice for prevention and management of delirium. Education of healthcare providers is recommended as a first step. Medication management such as appropriate prescribing, reduction of polypharmacy, and adequate treatment of pain plays a significant role in prevention and management of delirium. The full version of this guideline is available at www.geritricscareOnline.org.
The issue of delirium is discussed in more depth later in the textbook, in the chapter on Geriatric Psychology and the Injured Elderly.
Management of the Hospitalized Older Adults
The most dreaded consequence of hospitalization for an older adult, a fate that to some may be worse than death, is loss of independence and inability to return home. The possible development of long-term disability results from an interplay of pre-illness vulnerability, the acute stress of the illness or injury, and the treatment that is received in an acute care hospital.
More than 60 years ago, Dr. Sidney Katz noted a correlation between regaining basic functions (such as ability to bathe and dress independently) and the ability to return back home in older adults with hip fracture. This observation led to the creation of Katz’ Index of Activities of Daily Living (ADLs) which is still widely used as a measure of functional evaluation of older adults [98].
In his original article published in the Journal of the American Medical Association (JAMA) in 1963, Katz described “environmental artifacts”: “some patients were kept in bed either for convenience of the staff or due to restrictive safety policies” [98]. It has been demonstrated that inability to improve or regain function during hospital admission was associated not only with worse discharge outcomes but with increased mortality [99]. The process of aging itself causes some loss of muscle mass and strength and a decline in aerobic capacity [17]. Bed rest, whether intentional or unintentional, accelerates these processes. A study of bed rest in healthy older adults showed that 10 days of bed rest resulted in substantial loss of all measures of lower extremity strength and stair-climbing power and a 12 % loss of maximal aerobic capacity [100]; this effect is likely more marked in those who are frail. An older person admitted for observation for a small intracranial hemorrhage on Friday may be unable to wash, dress, or ambulate alone by Monday having spent most of the previous 72 hours in bed.
While bed rest after an injury may be a medical necessity, it is rarely the case. Patients in ICU setting are at higher risk for imposed immobility. Establishing an ICU early mobilization quality improvement program resulted in reduced ICU and hospital length of stay in three pilot institutions, and decreased rates of delirium and the need for sedation for the patients enrolled in the Johns Hopkins ICU early mobility program [101]. Details of the QI program and the tools can be found at www.iculiberation.org
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





