FLUIDS AND SODIUM
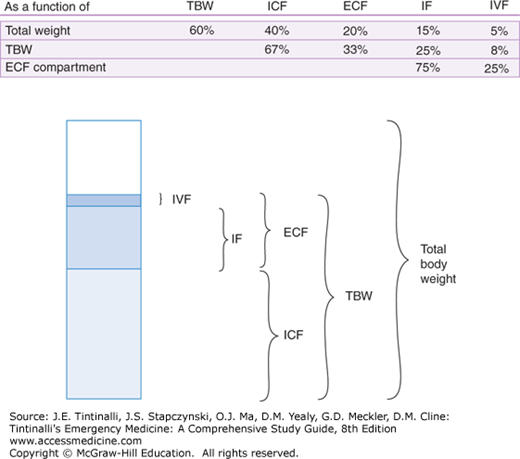
Total body water (TBW), which accounts for approximately 60% of total body weight, can be divided into intracellular fluid (ICF) and extracellular fluid (ECF) compartments. The ECF is comprised of intravascular fluid and extravascular, or interstitial, fluid. Body fluid compartment proportions for an adult are diagrammed in Figure 17-1. Figure 17-2 presents the individual characteristics of each compartment. Three fundamental homeostatic equilibriums govern the behavior of fluids: the osmotic equilibrium, the electric equilibrium, and the acid-base equilibrium.
The key point is that sodium is much more concentrated in the ECF (~140 mEq/L) than in the ICF (~10 mEq/L) but is equal in both compartments of the ECF because the capillary membrane between intravascular fluid and interstitial fluid is permeable to water and electrolytes. In contrast, the cell membrane is permeable to water but not to electrolytes, which are moved through ionic pumps against gradient to keep the intracellular sodium concentration constant around 10 mEq/L and potassium at 150 mEq/L. Table 17-1 lists the electrolyte concentration of body fluids and the most commonly used therapeutic solutions. Table 17-2 defines commonly used terms that describe measures or characteristics of electrolytes and/or disorders.
| Solution | Plasma | Interstitial | Intracellular | Normal Saline | Lactated Ringer’s Solution |
|---|---|---|---|---|---|
| Cations | |||||
| Sodium | 142 | 144 | 10 | 154 | 130 |
| Potassium | 4 | 4.5 | 150 | — | 4 |
| Magnesium* | 2 | 1 | 40 | — | — |
| Calcium† | 5 | 2.5 | — | — | 3 |
| Total cations | 153 | 152 | 200 | 154 | 137 |
| Anions | |||||
| Chloride | 104 | 113 | — | 154 | 109 |
| Lactate‡ | — | — | — | — | 28 |
| Phosphates | 2 | 2 | 120 | — | — |
| Sulfates | 1 | 1 | 30 | — | — |
| Bicarbonate | 27 | 30 | 10 | — | — |
| Proteins | 13 | 1 | 40 | — | — |
| Organic acids | 6 | 5 | — | — | — |
| Total anions | 153 | 152 | 200 | 154 | 137 |
| Term | Definition | Comments |
|---|---|---|
| Mole | 6.02 × 1023 molecules of a substance | Unit measure used in International System of Units format. |
| Equivalent | Mass (in grams) of a mole of a substance divided by charge of substance | Unit of measure used in conventional lab values. |
| Osmole | Amount of a substance (in moles) that dissociates to form 1 mole of osmotically active particles | |
| Osmolarity | Measure of solute concentration per unit volume of solvent | Osmolarity varies with changing temperature, because water changes its volume according to temperature. |
| Osmolality | Measure of solute concentration per unit mass of solvent | Osmolality is the preferred term because it remains constant with changes in temperature. |
| Tonicity or effective osmolality | Measure of the osmotic pressure gradient between two solutions, across a semipermeable membrane | Tonicity is affected only by solutes that cannot cross a semipermeable membrane. For example, tonicity is not affected by urea or glucose as they cross semipermeable membranes |
When two solutions are separated by a membrane that is permeable only to water, water crosses into the compartment with the more concentrated solution to equalize the ion concentration in each. The force driving this movement is “osmotic pressure.”1 In human fluids, the substances that contribute the most to osmotic pressure in ECF are Na+ and the anions HCO3– and Cl–, plus glucose. In physiology, this force is called effective osmolality or tonicity. This force is not affected by molecules like urea that may enter freely into the cells. The formula to calculate effective osmolality or tonicity is
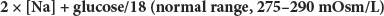
If values are in SI units, these are already molar (mmol/L, for example), so these do not need to be divided by their molecular weight.
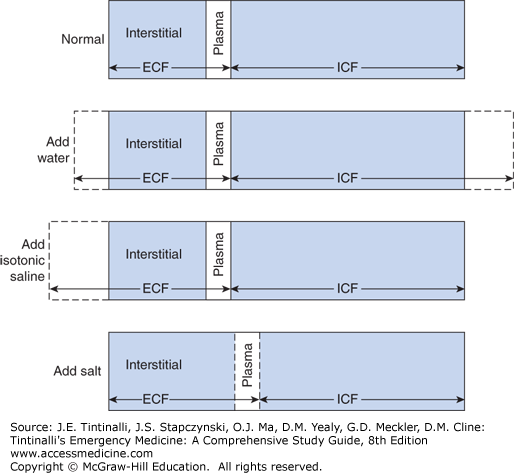
When 1 L of free water is added to the ECF it crosses the cell membrane into the ICF to equalize ECF osmolality. The result is TBW expansion and slight reduction in osmolality (Figure 17-3). When 1 L of isotonic saline solution 0.9% is added to the ECF, there is no movement of water into the cells, and the final result is ECF expansion only2 (Figure 17-3).
FIGURE 17-3.
Distribution of total body water into the intracellular fluid (ICF) and extracellular fluid (ECF) compartments. Addition of water expands both compartments. Addition of isotonic saline expands only the ECF, whereas addition of salt without water expands the ECF at the expense of the ICF. [Reproduced with permission from Eaton DC, Poole JP (eds): Vander’s Renal Physiology, 8th ed. McGraw-Hill, Inc., 2013. Fig 6-1.]
In contrast, when there is a fluid loss and a consequent increase in osmolality, the subject feels thirsty and the antidiuretic hormone (ADH) is secreted by the pituitary gland stimulated by baroreceptors and osmoreceptors, with a consequent urine water reabsorption and vasoconstriction.1,2,3
A useful index to roughly evaluate Na+ balance and the ability of the kidney to concentrate or dilute the urine is the electrolyte free water clearance (CH2Oe).2 It is expressed with the formula:
where Vurine is urine volume, UNa+ is the urine sodium level, UK+ is the urine potassium level, and PNa+ is the plasma sodium level. When more water is reabsorbed, CH2Oe is negative and hyponatremia will develop. When more water is excreted, CH2Oe is positive and hypernatremia will be present. The CH2Oe is calculated using a 24-hour urine collection, which is not possible in the ED. The spot urine calculation of the ratio, UNa+ + UK+/PNa+, is a reliable compromise. In hyponatremia, the ratio is >1, and in hypernatremia it is ≤0.5. A ratio between 0.5 and 1 is considered normal.
The human body tightly maintains serum [Na+] between 138 and 142 mEq/L despite what may be marked changes in daily intake depending on the person’s diet. Hyponatremia is a condition of excess water relative to Na+ and is defined as a serum [Na+] <138 mEq/L. However, symptomatic hyponatremia rarely occurs until [Na+] falls below 135 mEq/L or lower. In the setting of normal water intake, high circulating levels of ADH with subsequent water retention is a prerequisite for the development of hyponatremia.3 Urine osmolality is always >100 mOsm/L H2O with the exception of samples from patients with psychogenic polydipsia, which drives down urine osmolality below the typical minimum.
Mild hyponatremia is common, with an incidence of 15% to 30% in hospitalized patients; only 1% to 4% of patients have sodium levels below 130 mEq/L.4,5 Approximately 50% of cases are iatrogenic from administration of hypotonic fluids.4,5
The concentration of Na+ does not give information regarding volume status. Therefore, the first step in the evaluation should include a clinical evaluation of ECF volume status plus measured and calculated plasma osmolarities. In true hyponatremia, plasma osmolality is reduced; in factitious hyponatremic states, it is normal or increased as shown in Table 17-3.2
| Serum Osmolality | Clinical Conditions | Mechanisms |
|---|---|---|
| Hyperosmolality (Hypertonic hyponatremia) | Hyperglycemia Mannitol administration Glycerol administration Maltose administration | Hyponatremia due to osmotic diuresis |
| Iso-osmolality (Pseudohyponatremia) | Hyperproteinemia Hyperlipidemia | Displacement of serum water by elevated concentration of lipids or protein creating laboratory misinterpretation of normal [Na+] |
| Hypo-osmolality (Hypotonic hyponatremia) | See Table 17-4 | Hypervolemic Normovolemic Hypovolemic |
Hyperosmolar hyponatremia occurs when large quantities of osmotically active solutes accumulate in the ECF space. In this setting, there is a net movement of water from the ICF to the ECF, thereby effectively diluting the ECF [Na+]. This happens commonly with severe hyperglycemia. Each 100 milligram/dL increase in plasma glucose above the normal level of 100 milligrams/dL decreases the serum [Na+] by 1.6 mEq/L.1,2,3,4 Other causes of hypertonic hyponatremia are administration of osmotic agents like mannitol, glycerol, and maltose, causing an osmolar gap and hyponatremia. The osmolar gap is the difference between measured osmolality and calculated osmolality. Normally the difference is around 10 mOsm/L; if it is >15 mOsm/L it means that a nondetectable agent with osmotic activity is present, causing an osmolar gap. A consequent osmotic diuresis will cause [Na+] deficit with volume depletion that must be treated with saline solution. Other substances like methanol, alcohol, ethylene glycol, and urea, although causing an osmolar gap, do not cause water movement across membranes and therefore do not cause hyponatremia (see chapter 185, “Alcohols” in Toxicology section for more details).2
Pseudohyponatremia is a factitiously low value of [Na+] that may occur in the setting of severe hyperproteinemia or hyperlipidemia. This phenomena is due to displacement of serum water by an elevated concentration of lipids or protein creating laboratory misinterpretation of normal [Na+]; some laboratories use instruments that avoid this laboratory error; check with your laboratory administrator. Patients are asymptomatic; treatment is not needed.
In Table 17-4,6 the different causes of hypo-osmolar (hypotonic) hyponatremia according to volume status are listed.2,3 Hyponatremia develops due to an increase in ADH secretion and activity, which causes impaired water excretion and increased water reabsorption. In situations like heart failure,7,8 cirrhosis,9 and nephrotic syndrome, the effective arterial blood volume is decreased because water is mainly distributed to the interstitial space. Thus Na+ and water reabsorption are increased, and water excretion is reduced.
| Clinical Conditions | Orthostatic Hypotension | Edema | U[Na+], mEq/L* | Uosm, mOsm/L* | |
|---|---|---|---|---|---|
| Hypervolemic hypernatremia | CHF Cirrhosis Nephrotic syndrome Acute and chronic kidney disease | Absent | Yes | Compensated: >20 Decompensated: <10 | Compensated: <100 Decompensated: >100 |
| Normovolemic hyponatremia | Psychogenic polydipsia Glucocorticoid deficit Hypokalemia Drugs SIADH | Absent | No | >20 | >100 |
| Renal hypovolemic hyponatremia | Diuretics Mineralocorticoid deficit Salt-losing nephropathy | Normally present | No | >20 | >100 |
| Extrarenal hypovolemic hyponatremia | Vomiting Diarrhea | Normally present | No | <10 | >100 |
Two important hyponatremic disorders are the syndrome of inappropriate ADH secretion and the less common cerebral salt-wasting syndrome.1,2 Both conditions are diagnoses of exclusion after dismissing other causes of hyponatremia. The onset of both syndromes is linked to chronic cerebral disease, but syndrome of inappropriate ADH secretion may also be caused by many other diseases and conditions as described in Table 17-5. In syndrome of inappropriate ADH secretion, volume status is normal, whereas in cerebral salt-wasting syndrome, there is hypovolemia; therefore, these two disorders are treated differently (see treatment section below).
|
Methylenedioxymethamphetamine (MDMA or Ecstasy) intoxication is an uncommon but important cause of hyponatremia that may be profound. This “club drug” induces inappropriate secretion of ADH and causes increased gut water absorption10 (see also chapter 188, “Hallucinogens”).
The most important symptoms of hyponatremia are due to its effects on the brain; symptoms can be divided into moderately severe and severe, according to a European clinical practice guideline.3,4 Moderately severe symptoms often start when a plasma [Na] is <130 mEq/L and consist of headache, nausea, disorientation, confusion, agitation, ataxia, and areflexia. When [Na+] reach levels <120 mEq/L, severe symptoms may develop including intractable vomiting, seizures, coma, and ultimately respiratory arrest due to brainstem herniation. Brain injury may become irreversible. The symptoms of hyponatremia can be due to many other conditions, and clinicians are cautioned to consider other etiologies before making treatment decisions.4 The presence of hyponatremia-related symptoms is directly related to the rapidity of onset. After a certain period, brain cells begin to adapt to hyponatremia. Initially the hypo-osmolality drives water into the brain cells yielding swelling.2,3 Due to the rigid skull, intracranial hypertension occurs and the described symptoms begin. After 48 hours, the brain cells start to adapt by extruding Na+, K+, Cl–, and organic osmolytes like glycine and taurine from the cells, reducing cell osmolality and preventing further water uptake. In several clinical or physiologic conditions, this adaptation mechanism is impaired, as in the syndrome of inappropriate ADH secretion, in children, in menstruating women, and in hypoxia. In such cases, symptoms are more severe and persistent.
The diagnosis of hyponatremia and its subtypes is based on the clinical findings of volume status in association with specific laboratory values including serum [Na+], serum osmolality, volume status, urinary sodium (UNa+), and urine osmolality (Uosm). Acute and chronic hyponatremia are defined by an onset time of less than (acute) or greater than (chronic) 24 to 48 hours. Experts recommend that when duration is unknown, the hyponatremia should be assumed to be chronic and treated as chronic with a longer correction time. If urine osmolality is not readily available from the laboratory, it can be estimated using urinary specific gravity (π). Consider the numerals in the hundredths and thousandths decimal places of the π as whole numbers and multiply them by 35 to obtain Uosm. As an example, for a π of 1.005, Uosm = 05 × 35 = 175 mOsm/L.1 Table 17-4 lists the values of UNa+ and Uosm in different classifications of hyponatremia according to volume status and the differential diagnosis for each classification. As a rule, only in patients with edematous syndromes and in patients with vomiting and diarrhea will UNa+ be found to be <10 mEq/L.6 The diagnostic criteria for syndrome of inappropriate ADH secretion are listed in Table 17-6.
| Diagnostic Criteria |
|---|
Hypotonic hyponatremia with (Posm <275 mOsm/kg H2O) Inappropriately elevated urinary osmolality (usually >200 mOsm/kg) Elevated urinary [Na+] (typically >20 mEq/L) Clinical euvolemia Normal adrenal, renal, cardiac, hepatic, and thyroid functions |
Use care when assessing patients with potential exercise-associated hyponatremia. Since the worldwide effort to encourage consuming fluids during endurance exercise beginning in the early 1980s, overhydration with hypotonic fluids is now being seen. If a postexercise athlete presents with bloating, nausea, vomiting, and edema (check wrists and fingers), consider hyponatremia; dehydration presents with excessive thirst, sunken eyes, poor skin turgor, and postural hypotension.
Treatment of hyponatremia is guided by four variables: severity of symptoms, rate of onset, volume status, and the current serum [Na+]. When [Na+] is <120 mEg/L, the patient presents with severe neurologic symptoms, and hyponatremia is acute, the initial treatment includes infusion of 3% hypertonic saline as recommended by European guidelines,4 and U.S. experts5,11 (Table 17-7).
| Step 1 | Assess for indication for 3% hypertonic saline: severe symptoms of hyponatremia such as seizures or coma with suspected impending brainstem herniation in setting of acute* or chronic† hyponatremia |
| Step 2 | Infuse 100 mL of 3% hypertonic saline IV over 10–15 min‡ |
| Step 3 | Measure serum sodium level after each 3% hypertonic saline infusion |
| Step 4 | Stop infusion when symptoms improve, or a target of 5 mEq/L (range 4–6 mEq/L) increase in serum sodium concentration is achieved. |
| Step 5 | May repeat 100 mL of 3% hypertonic saline up to three total doses, or a total of 300 mL IV of 3% hypertonic saline. |
| Step 6 | Keep the IV line open with minimal volume of 0.9% normal saline until cause-specific treatment is started. Limit increase in sodium level to no more than 8 mEq/L during the first 24 h. |
Raising serum sodium by 4 to 6 mEq/L is typically all that is required to see an improvement in severe neurologic symptoms.4,11,12 The volume of a saline solution required to raise the serum sodium the desired amount can be calculated. First calculate the expected change in serum sodium from the infusion of a liter of saline solution. Second, determine the portion of the liter required to raise the sodium the desired amount. To calculate the expected change in serum sodium after an infusion of 1 L IV saline, use the following formula:

Estimate TBW based on age, sex, and weight of the patient. For children and adult males <65 years old, TBW is 60% of the weight; for adult females <65 years old and elderly males, TBW is 50% of the weight; for elderly females, TBW is 45% of weight. Three percent hypertonic saline contains 513 mEq/L of sodium. As an example, a clinician desires to raise the sodium level of a 68-year-old male having seizures secondary to a [Na+] of 108 mEq/L, using 3% hypertonic saline. The man weighs 70 kg; therefore, the calculated TBW would be [0.5correction factor × 70 kg] = 35 L. Change in [Na+] expected = (513 – 108)/(35 + 1) = 11.25 mEq rise in serum sodium if the man was given 1000 mL of 3% hypertonic saline. The fraction of the liter of 3% hypertonic saline required to raise serum sodium 5 mEq/L would be 444 mL of 3% hypertonic saline.
After clinical improvement in severe symptoms, the 3% hypertonic saline must be stopped and either continued at a slower rate or with a different [Na+] in the solution. For patients with acute hyponatremia with mild or moderate symptoms, 3% hypertonic saline infusion at 0.5 to 2 mL/kg/h can be given with frequent [Na+] checks every 2 hours. Na+ and K+ lost in urine should be replaced with the appropriate solution.1,2
In chronic hyponatremia, the [Na+] correction should be slower than for acute hyponatremia. Rapid correction increases risk for the most dangerous complication of treatment, the osmotic demyelination syndrome. For chronic hyponatremia [Na+], the correction rate should not exceed 6 mEq/24 h in high-risk patients and 12 mEq/24 h in low-risk patients (see “Osmotic Demyelination Syndrome” section below for risks).3,4 Hypertonic (3%) saline can be given at a low infusion rate, 0.5 to 1 mL/kg/h, with frequent [Na+] checks. Isotonic (0.9%) saline is frequently used (sometimes before the [Na+] is known), especially for the treatment of mild hyponatremia; however, the additional fluid load must be accounted for in treatment calculations. Loop diuretics (primarily furosemide, starting with a small dose of 20 milligrams IV) may be used in addition to treatment with saline infusions. Urine volume and [Na+] should be strictly measured. Specific recommendations for hyponatremia treatment are summarized in Table 17-8.2
| Clinical Condition | Therapy | Cautions/Comments |
|---|---|---|
| Chronic heart failure and cirrhosis | Loop diuretics, water restriction. Consider vasopressin antagonists* if the above therapies fail for patients with chronic heart failure. | When vasopressin antagonists* are used serum [Na+] should be frequently measured to avoid hypernatremia. FDA recommends against vasopressin antagonists in patients with liver disease. |
| Nephrotic syndrome | Water restriction | |
| Acute or chronic kidney disease | Water restriction | Frequent assessment of creatinine |
| Psychogenic polydipsia | Water restriction | Treat the underlying psychiatric disease |
| Hypothyroidism | Levothyroxine | Several days of therapy are typically required to correct hyponatremia. |
| Glucocorticoid deficiency | Hydrocortisone. If neurologic symptoms, consider vasopressin antagonists* if resistant to hydrocortisone. | When vasopressin antagonists* are used, [Na+] should be frequently measured. |
| SIADH | Water restriction. Enhanced Na+ and protein intake + furosemide. Vasopressin antagonists* can be used for [Na+] <125 mEq/L. Demeclocycline. Lithium. | Isotonic (0.9%) NaCl may worsen hyponatremia; when vasopressin antagonists* are used, [Na+] should be frequently measured. |
| Diarrhea and vomiting | Isotonic (0.9%) NaCl. Add KCl if hypokalemia is present. | Treat the cause, monitor hemodynamic status |
| Diuretics (most commonly thiazides) | Stop diuretic. KCl may be sufficient in patients with coexistent potassium depletion and normal dietary sodium intake. NaCl can be given orally. | Slow correction is recommended. Do not overcorrect K+ deficit. |
| Mineralocorticoid deficiency | Replace volume deficit. Fludrocortisone therapy is indicated once diagnosis is confirmed. | Mechanism: volume depletion → ↑ ADH → decreases water excretion, ↑ Na loss |
| Salt losing nephropathies | Isotonic (0.9%) NaCl. | |
| Cerebral salt wasting | Isotonic (0.9%) NaCl. Fludrocortisone may be considered after the diagnosis is confirmed. | NaCl orally at home |
Osmotic demyelination syndrome is caused by rapid correction of hyponatremia (>12 mEq/L/24 h) as water moves from cells to ECF yielding intracellular dehydration (Figure 17-4). Risk factors for osmotic demyelination syndrome include [Na+] <120 mEq/L, chronic heart failure, alcoholism, cirrhosis, hypokalemia, malnutrition, and treatment with vasopressin antagonists such as tolvaptan. Main symptoms are dysarthria, dysphagia, lethargy, paraparesis or quadriparesis, seizures, coma, and death. The treatment of [Na+] overcorrection is rarely done in the ED but consists of giving 5% dextrose in water at 3 mL/kg/h, loop diuretics, and desmopressin.3,4
Hypernatremia is defined as serum or plasma [Na+] >145 mEq/L and hyperosmolality (serum osmolality >295 mOsm/L).
Hypernatremia results from a deficit in TBW and/or a net gain of Na+ (less common). When [Na+] and osmolality increase, normal subjects become thirsty, drink free water, and the Na+ level returns toward normal. Any clinical situation that impairs the patient’s sense of thirst, limits the availability of water, limits the kidney’s ability to concentrate urine, or results in increased salt intake predisposes the patient to hypernatremia. Elderly patients, decompensated diabetics, infants, and hospitalized patients are at particular risk of developing hypernatremia. In addition, hypernatremia may be the result of loss of free water in diarrheal stools or in the urine.13
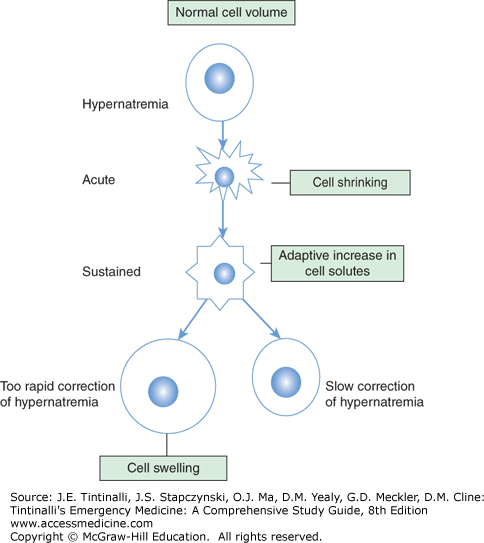
As in hyponatremia, symptoms will be more severe and evident when the onset is rapid; after the first 48 hours, there is an adaptation of brain cells with an increase in electrolytes and organic osmolytes and thus increased intracellular water partly correcting the initial cell shrinking (Figure 17-5).
If severe hypernatremia develops in the course of minutes to hours, such as from a massive salt overdose in a suicide attempt, a suddenly shrinking brain may prompt intracranial hemorrhage. While the causes of hypernatremia are many, leading to varied signs and symptoms, the most serious manifestations are related to changes in the brain. If hypernatremia is corrected too rapidly, cerebral edema and potentially central herniation may occur.
Based on volume status, hypernatremia may be classified as hypovolemic hypernatremia (decreased TBW and total body Na+ with a relatively greater decrease in TBW), hypervolemic hypernatremia (increased total body Na+ with normal or increased TBW), or normovolemic hypernatremia (near normal total body sodium and decreased TBW)1,2 (Table 17-9).
| Clinical Conditions | Orthostatic Hypotension | Edema | U[Na+], mEq/L | Uosm, mOsm/kg H2O | |
|---|---|---|---|---|---|
| Hypervolemic hypernatremia | Cushing’s syndrome Primary hyperaldosteronism Salt water intake Iatrogenic | Absent unless treated with diuretics | Yes | Compensated >20 | >100 |
| Normovolemic hypernatremia | DI Central DI Partial DI Gestational DI Nephrogenic DI Hypodipsia | Absent | No | >20 | Central DI <300 Partial DI >300 but <800 |
| Renal hypovolemic hypernatremia | Osmotic diuretics Loop diuretics Postobstructive diuresis | Normally present | No | >20 | >100 |
| Extrarenal hypovolemic hypernatremia | Vomiting Diarrhea GI fistulas Sweating Burns | Normally present | No | <10 | >800 |
History depends on hypernatremia type and may reveal nausea and vomiting, lethargy, weakness, increased thirst, low water intake, salt intake, polyuria (>3000 mL of urine/24 h), diabetes, hypercalcemia, hypokalemia, medications such as lactulose, loop diuretics, lithium, demeclocycline (may cause nephrogenic diabetes insipidus), or nonsteroidal anti-inflammatory drugs (may cause interstitial nephritis). Physical exam may reveal hypotension, tachycardia, orthostatic blood pressures, sunken eyes, dry mucous membranes (symptoms of hypovolemia) altered mental status (may be present in any the hypernatremia classifications), poor skin turgor, or edema in hypervolemic hypernatremia (Table 17-9). Without intervention, coma, seizures, and shock may occur. Signs of Cushing’s syndrome may be present, including moon facies, fatty deposits between the shoulders and upper back, and thinning of the skin.
The diagnosis of hypernatremia and its classification are based on the clinical evaluation including volume status and specific laboratory tests, including serum electrolytes and osmolality, urine osmolality, urea/creatinine ratio, and free water deficit. A BUN/creatinine ratio >40 is indicative of hyperosmolar dehydration. The free water deficit1,2 may be calculated with the formula:
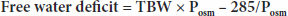
where TBW is calculated using age and sex (giving a correction factor for body water) times weight in kilograms (see hyponatremia treatment section for scale), Posm = 2 × [Na+] + glucose/18, and 285 is used as normal plasma osmolality. As an example, a 60-kg woman with a [Na+] of 165 mEq/L and glucose of 130 milligrams/dL has a free water deficit calculated as follows: TBW = 0.5 × 60 = 30; Posm = (2 × 165 = 330) + (130/18 = 7.2) = 337.2 mOsm/kg H2O; free water deficit = 30 × (337.2 – 285)/337.2 = 4.64 L. Urine osmolality can be used to suggest the type of hypernatremia (Table 17-10). If urine osmolality is not readily available, it can be estimated using urine specific gravity (π). Consider the numerals in the hundredths and thousandths decimal places of the π as whole numbers and multiply them by 35 to estimate Uosm.
| Urine Osmolality (Uosm) | Potential Hypernatremic State |
|---|---|
| Uosm <300 mOsm/kg H2O | Central or nephrogenic diabetes insipidus |
| Uosm >300, <800 mOsm/kg H2O | Partial diabetes insipidus or osmotic diuresis |
| Uosm >800 mOsm/kg H2O | Hypertonic dehydration |
First, shock, hypoperfusion, or volume deficits should be treated with isotonic (0.9%) saline. Second, treat any existing underlying cause, such as diabetes insipidus (see “Diabetes Insipidus” section below) vomiting, diarrhea, or fever. Third, correct the patient’s free water deficit at a rate reflecting the acuity or duration time of the hypernatremia onset (Table 17-11).11,14,15 In cases of a lethal sodium chloride ingestion/load (0.75 to 3.0 grams/kg) less than 6 hours prior to presentation, FWD may be replaced rapidly with no reported adverse events.11,14 Management requires evaluating volume status and the free water deficit (see formula above in diagnosis section). When the adaptation of brain cells is incomplete (onset over <48 hours), the correction rate of acute hypernatremia can be performed at a rate of 1 mEq/L/h. In an alert patient capable of safely drinking water, the route of administration should be two-thirds free water orally and one-third IV. If hypernatremia is chronic (onset over >48 hours), the rate of correction should be slower, to avoid the risk of cerebral edema, at no more than 0.5 mEq/L/h or 10 to 12 mEq/24 h.11,16
| Treatment | Indication and Comments |
|---|---|
| Isotonic (0.9%) saline | Use for correction of volumn deficits. |
| Etiology-specific therapy | Treat fever with antipyretics, vomiting with antiemetics, and diabetes insipidus with desmopressin (see “Diabetes Insipidus” section below). |
| D5W to replace free water deficit over 2-3 days | In cases of chronic hypernatremia, it is suggested that correcting (lowering) the sodium level should occur at a rate of no more than 0.5 mEq/L/h or 10 to 12 mEq/24 h. |
| 0.45% normal saline at 100 mL/hour | Correct volume deficts first. A commonly used infusion for mild to moderate hypernatremia, but this therapy adds to total body sodium. |
| D5W to replace free water deficit over 1-2 hours | Reserved only for those cases where acuity is known to be <6 h and the salt load is known to be lethal (0.75–3.0 grams/kg of body weight). |
| Hemodialysis | An alternative or as a suppliment to D5W to replace free water deficit in life-threatenting acute cases of salt ingestion. |
Diabetes insipidus is a disease where the ability of the kidney to reabsorb free water is compromised.2,17 The disorder is characterized by polyuria, polydipsia, and an increased volume of hypo-osmolar urine. Hypernatremia is present only when the thirst center is impaired or water intake is reduced. Diabetes insipidus can be central (also called neurogenic), due to inadequate ADH secretion, or renal (also called nephrogenic), when ADH secretion is normal or increased but the v2R receptors of the kidney’s collecting duct cells do not respond appropriately to ADH. Diabetes insipidus may be congenital or acquired. In Table 17-12, the main causes of diabetes insipidus are listed. Congenital forms of diabetes insipidus present during infancy. Eventually, recurrent cellular dehydration causes cerebral calcifications that manifest as delayed intellectual advancement.
| Class | Acquisition | Pathophysiology |
|---|---|---|
| Central or neurogenic diabetes insipidus | Congenital | Structural malformations affecting the hypothalamus or pituitary Autosomal dominant (or rarely recessive) mutations in the gene encoding AVP-NPII precursor protein |
| Acquired | Primary tumors (craniopharyngioma) or metastases Infection (e.g., meningitis, encephalitis) Histiocytosis and granulomatous diseases Trauma Surgery Idiopathic | |
| Nephrogenic diabetes insipidus | Congenital | X-linked: inactivating mutations in AVPR2 gene Autosomal: recessive or dominant mutations in AQP-2 gene |
| Acquired | Primary renal disease Obstructive uropathy Metabolic causes (e.g., hypokalemia, hypercalcemia) Sickle cell disease Drugs (e.g., lithium, demeclocycline) | |
| Primary polydipsia or dipsogenic diabetes insipidus | Acquired | Psychogenic illness characterized by excessive fluid intake. Treatment is aimed at the psychiatric disease. |
Central diabetes insipidus is acquired in most cases, associated with various disorders that cause destruction of ADH-secreting neurons. When a diagnosis is not possible, despite imaging and other diagnostic tests, diabetes insipidus will be defined as idiopathic diabetes insipidus.
The most common clinical symptoms and signs are excessive thirst, polydipsia, and polyuria plus several nonspecific symptoms including weakness, lethargy, myalgias, and irritability. In infancy, congenital forms of diabetes insipidus present with fatigue and weakness often manifested by less activity or tiring with feeding, vomiting, polyuria, and sometimes fever. Diagnosis can be suspected in the ED by clinical presentation, but the diagnosis requires a prolonged test, requiring 4 to 18 hours. Urine osmolality is assessed after water deprivation; many cases require another assessment after a dose of desmopressin, the “water deprivation test.” A spot check in the ED without water deprivation will typically reveal a Uosm of <300 mOsm/L. In central diabetes insipidus, a cerebral MRI is indicated (on a nonurgent outpatient basis) to evaluate the hypothalamic–pituitary area (Figure 17-6).
FIGURE 17-6.
MRI image of a craniopharyngioma that caused diabetes insipidus in a 46-year-old patient.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree