W13 Fiberoptic Bronchoscopy
 Before Procedure
Before Procedure
Indications
• Diagnostic uses:
• To evaluate problems associated with endotracheal tubes and airway stents (e.g., tracheal damage, device malposition, airway obstruction)
• To obtain lower respiratory tract specimens by bronchoalveolar lavage (BAL) or protected specimen brushing (PSB) for cytologic or microbiological analyses
• To determine the location and extent of respiratory tract injury after toxic inhalation or aspiration of gastric contents
Equipment
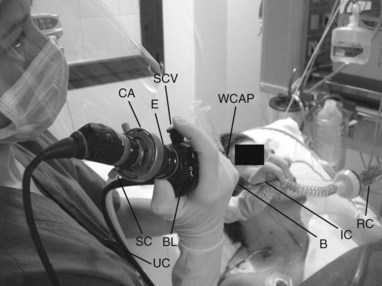
• Fiberoptic bronchoscope made up of a few components which are incorporated into a functional unit:
• Control handle; it contains:
• Eyepiece to which video or photographic devices may be attached. Just under the lens, a diopter adjustment ring is used to focus and adjust the eyepiece to fit each individual’s eyesight. In some bronchoscopes, the top of the endoscopic view is marked by an indent or black triangle to assist in orientation.
• Bending lever; located on the back of the handle and used to activate the up-and-down movement of the last 2 to 3 cm of the insertion cord
• Insertion cord: the flexible bronchoscopic element that hangs from the control handle and is introduced into the airways. Within it are the viewing bundle, one to three light bundles, the working channel, and two wires to control the distal end of the scope.
• Universal cord: arises from the side of the control handle and transmits light from the light source to the endoscope and then down to the insertion cord to illuminate the field of view. The light source is a metal box to which the universal cord attaches. In some types of bronchoscopes (portable), the universal cord is not needed, as the light source is built into the control handle of the instrument, and power is provided by a battery system. Modern digital flexible endoscopic systems use a charge-coupled device chip placed at the end of the scope to relay digitized information to the monitor via a processor. The venting connector is a component of the bronchoscope, usually located on the universal cord. The ethylene oxide sterilization venting cap and leakage tester are attached to this connector. The ethylene oxide cap must be installed when the endoscope is subjected to gas sterilization and during transportation by air; it must be removed prior to immersion or when the instrument is in use.
• Ancillary technical materials:
• Antifogging systems, including warm water (max 60°C), weak soap solutions, and commercially available antifogging solutions. When fogging occurs during procedure, bringing the tip of the endoscope into contact with the mucosal surface may eliminate lens fogging.
• Microbiology and cytology brushes, flexible forceps, retrieval valves, transbronchial aspiration needles, fixatives
• Specimen-collection traps, syringes for medication delivery, normal saline solution for bronchoalveolar lavage
• Oral intubating airway that provides an open air space in the oropharynx and protects the endoscope from being bitten by the patient; it is useful if the oral route is chosen in patients under general anesthesia.
• Endoscopy mask to assist fiberoptic intubation in patients being ventilated by face mask; provided with a rubber diaphragm that permits passage of either the endoscope or tracheal tube into the airways and prevents air leaking
• Laryngeal mask airway or other extraglottic devices in various sizes for ventilator support in emergency situations. Laryngeal mask airway may also be used to aid passage of the bronchoscope into the trachea.
• Adapter for insertion of the bronchoscope into the airways while preventing loss of respiratory gases and maintaining ventilation and positive end-expiratory pressure throughout procedure during either invasive or noninvasive ventilation (NIV) (Figures W13-1 and W13-2)
• Self-inflating bag or anesthesia bag attached to a face mask by a T-adapter, for bag-mask ventilation in nonintubated, sedated patients undergoing bronchoscopy
• Medications:
• Lidocaine for topical anesthesia. The minimum amount of lidocaine necessary should be used when instilled through the endoscope; the total dose should be limited to 8.2 mg/kg in adults. Great care must be given when administering lidocaine to patients with liver or cardiac failure
• Synthetic narcotics such as fentanyl or alfentanil to provide sedation and analgesia and suppress cough reflex
• Monitoring devices:
• Continuous intraarterial blood pressure or intermittent cuff blood pressure measurement at least every 5 minutes
• Respiratory function monitor in patients under mechanical ventilation, for monitoring ventilation parameters such as exhaled tidal volume and peak inspiratory pressure
• Cleaning and sterilization equipment:
• Dedicated room for cleaning and manual or automated sterilization; automated washer disinfectors are recommended to minimize staff contact with disinfectant and their fumes.
• Soft nonabrasive cleaning cloth to gently wipe the external surfaces and components of the endoscope immediately after use
• Water or neutral detergent solution to irrigate all accessible channels of the endoscope at the end of the procedure
• Cleaning brushes and neutral detergent solution for a thorough cleaning of internal and external surfaces of the endoscope. Cleaning brushes are passed through the working channel access port, the suction port opening, and the suction connector; they are also used to carefully clean the distal end of the instrument. The brush head should be cleaned each time it emerges from the endoscope.
• High-level disinfection or sterilization agent such as peracetic acid, glutaraldehyde, or ethylene oxide
• 70% ethyl or isopropyl alcohol to flush the external surfaces and inner channels of the endoscope when the quality of the rinse water is in doubt or to assist in the drying process
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree