6. Individualized risk assessment determines choice of modality. The risk of iatrogenic premature delivery, prompted by false-positive testing results, must be considered.
B. Screening ultrasound survey
1. A screening ultrasound exam is commonly performed at 16 to 20 weeks and sometimes during the first trimester27 in order to:
a. Determine the number of fetuses
b. Estimate fetal age
c. Identify placental location
d. Assess fetal anatomy to detect any anomalies or problems
2. Benefits include improvement in:
a. Estimation of gestational age, to decrease the risk of postterm pregnancy
b. Detection of aneuploidy (e.g., Down syndrome)
c. Identification of multiple gestations
d. Detection of congenital anomalies27
C. Maternal monitoring of fetal movement is available to all women, without need for technology, and is recommended beginning at 26 to 32 weeks for all pregnancies with risk factors for adverse perinatal outcomes.3
1. Healthy women without risk factors may benefit from being aware of the significance of fetal movements during the third trimester and should perform a fetal movement count if they perceive less movement.3
2. The best movement-counting protocol (number of movements; duration of counting) has not been established. Hence, there are a number of protocols in use at present.1
D. NST uses EFM to assess for temporary FHR accelerations, which accompany spontaneous movement in a fetus that is either acidotic or neurologically depressed.
1. NST is categorized as reactive (i.e., reassuring) if the fetus has two or more accelerations (≥15 beats per minute [bpm] increase, lasting ≥15 seconds) within 20 minutes.
2. NST is characterized as nonreactive if there are less than two accelerations in 40 minutes.
3. If there are no fetal movements, vibroacoustic stimulation may be applied for 1 to 2 seconds to arouse a sleeping fetus to elicit FHR accelerations, which are predictive of fetal well-being.
4. NST relies on an intact fetal autonomic nervous system—fetal movement is accompanied by increased sympathetic output, accounting for FHR acceleration. These neural pathways do not mature until 28 weeks’ gestation. Prior to 28 weeks, 50% of NSTs may be nonreactive and 15% prior to 32 weeks. This is due to autonomic nervous system immaturity.
5. Lower thresholds (≥10 bpm rise, ≥10 seconds) for deeming the NST reactive appear to preserve the predictive value of a normal test. In addition to reactive/nonreactive categorization of NST results, the other basic parameters of EFM are assessed (baseline rate, variable, decelerations).
6. If uterine contractions are present, then a NST is technically a spontaneous CST, although contractions may not be frequent enough to qualify as a formal CST.
7. Although a reactive NST is reliable in confirming fetal well-being (i.e., a high negative predictive value [NPV]), a nonreactive NST falsely predicts fetal hypoxemia/acidosis 55% to 90% of the time.28–31
8. An abnormal result prompts a stepwise approach to further fetal assessment, typically a CST or BPP. Depending on the clinical situation, in utero resuscitation, or even expedited fetal delivery, may be indicated.
E. CST, also known as oxytocin challenge test (OCT) was first described in 1972.
1. It is a means of providing a physiologic stress to the fetus, the response to which may reflect the degree of fetoplacental respiratory reserve.32
2. Uterine contractions normally reduce uterine perfusion and fetal oxygen delivery, such that a fetus with diminished uteroplacental reserve may develop hypoxia and/or asphyxia sufficient enough to cause alterations in fetal compensatory physiologic parameters.3
3. A NST is performed before a CST. During the latter, the patient is placed in the lateral recumbent position, continuous EFM is performed, and intravenous (IV) oxytocin is titrated to effect an adequate contraction pattern (at least three contractions of at least 40 seconds duration each, within 10 minutes).
4. Nipple stimulation (woman rubs one nipple with her fingers, through her clothes, rapidly, but gently, for 2 minutes, then stops for 5 minutes) is equally or more successful in inducing adequate contractions for a CST, compared to exogenous oxytocin administration.
5. CST results are classified as follows:
a. Negative: normal FHR baseline rate with no late or significant variable decelerations
b. Positive: late FHR decelerations occurring with >50% of induced contractions (even if contraction frequency is <3 per 10 minutes)
c. Equivocal-suspicious: intermittent late FHR decelerations, or significant variable FHR decelerations
d. Equivocal: FHR decelerations occurring with contractions more frequent than two per 10 minutes, or lasting >90 seconds.
e. Unsatisfactory: less than the requisite contraction rate of three per 10 minutes, or uninterpretable cardiotocography.
6. Like the NST, a CST has excellent NPV but poor PPV for perinatal morbidity (8.7% to 14.9%).
7. CST has been supplanted by newer methods (i.e., assessment of biophysical variables and vascular flow measurements) but is still used for many at-risk fetuses—preterm, postdates, uteroplacental pathology, growth restriction.
8. The only contraindications include those that would preclude labor or vaginal delivery and a gestational age (<24 weeks) that would preclude intervention if the result were abnormal.
F. Biophysical profile. BPP combines the NST with sonography to assess current fetal well-being.
1. Sonography is performed over 30 minutes, assesses fetal behaviors (fetal movement, tone, breathing movements), and measures amniotic fluid volume.
2. BPP serves as a sensitive method of detecting acute or chronic fetal hypoxia.
3. Each of five assessed components is assigned 2 points if criteria (outlined in Table 7.2) are met, or 0 if they are not. A composite score is calculated: 8 to 10 is normal, 6 is equivocal, and 4 or less is abnormal.

4. Fetal adaptive responses to hypoxia include redirection of blood flow to the fetal brain and heart, but away from fetal kidneys. As a result, renal blood flow and urine output decrease, resulting in oligohydramnios as assessed by amniotic fluid volume.3
5. Ultrasonography is used to measure amniotic fluid volume in four intrauterine quadrants and calculate the amniotic fluid index (AFI). Alternatively, the maximal vertical pocket (MVP) is measured.
6. Oligohydramnios is defined as either AFI <5 cm or MVP <2 cm. Both methods appear to be equivalent in predicting adverse outcomes, but use of AFI is associated with higher rates of cesarean delivery and induction of labor without improved perinatal outcome.
7. A “modified BPP” (mBPP), which consists of only NST (short-term indicator of fetal hypoxia/acidemia) and AFI (indicator of long-term placental insufficiency), is simpler and quicker to perform. Its effectiveness in identifying a compromised fetus is similar to BPP.
8. BPP and mBPP both have an NPV of >99% (0.8 stillbirths per 1,000 reassuring BPP or mBPP results).15 Progressively lower BPP test scores correlate directly with fetal acidemia, measured by umbilical vein (UV) pH. BPP scores of 8 to 10 are not associated with fetal acidemia, a score of 6 is equivocal, and 0 to 4 is highly associated with fetal acidemia.33–35
9. BPP scores are inversely related to perinatal morbidity, as measured by five outcome variables.
a. Fetal decelerations prompting operative obstetric intervention
b. Five-minute Apgar score <7
c. UV pH <7.20
d. Fetal growth restriction (FGR) (<10% percentile for gestational age)
e. Admission to the neonatal intensive care unit (NICU)
10. Perinatal mortality (total, and corrected for major fetal anomaly) exhibits a significant inverse exponential relationship to BPP scores without intervention.33
11. BPP results must be interpreted in context.
a. First, the test should be performed when indicated (see Table 7.1) and at an appropriate gestational age (not before 32 0/7 weeks for most at-risk patients).
b. In the case of a nonpersistent indication (e.g., one episode of decreased fetal movement) and reassuring testing, then repeated surveillance testing is not necessary.
c. If the risk factor that prompts testing persists, but testing is reassuring, then surveillance testing is typically repeated weekly, although it may be performed more frequently for some high-risk conditions.
d. An abnormal result is considered in clinical context. Abnormal results associated with acute maternal conditions often normalize once the maternal condition is corrected.
e. Abnormal BPP results typically prompt further evaluation, to minimize risk of unnecessary premature delivery based on one false-positive result.
f. Contributing to high false-positive results are various benign factors, such as fetal sleep, hypoglycemia, supine hypotension syndrome, and maternally administered opioids and sedatives.
G. Doppler velocimetry is a noninvasive assessment technique used in conjunction with other fetal surveillance modalities, specifically when FGR is suspected.
1. In normal pregnancy, trophoblasts invade the media of maternal spiral arteries and transform them into high-flow, low-resistance shunts.3 In parallel, villous development (sprouting and differentiation) reduces resistance in the fetal circulation through the placenta.13 Consequently, both maternal uterine arterial and fetal umbilical arterial flows increase early in pregnancy and continue to increase throughout normal pregnancy. They are characterized by high diastolic flow, and Doppler velocimetry flow indices are commonly used to assess them, including:
a. Systolic-to-diastolic ratio (S/D): ratio of frequency shift during systole to that during diastole; commonly used to evaluate fetal UA flow
b. Pulsatility index (PI): difference between frequency shifts during systole and diastole, divided by mean frequency shift; commonly used to evaluate maternal uterine arterial flow and fetal middle cerebral artery (MCA) flow
c. Resistance index (RI): difference between frequency shifts during systole and diastole, divided by frequency shift during systole; most often used to assess MCA flow
2. Many conditions (e.g., hypertensive disorders, diabetes mellitus, collagen vascular disease, chronic placental abruption, placental infarction, thin or circumvallate placentas) may interfere with normal placental vascular development, thereby jeopardizing gas exchange and nutrient delivery.
3. Placental dysfunction is associated with higher vascular resistance and uteroplacental insufficiency, that is, reduced blood flow to the fetus, risking growth restriction. Increased resistance reduces diastolic flow to a greater degree than systolic flow, increasing all three Doppler index values.
4. There is ongoing research into the usefulness of Doppler velocimetry measurements of fetal MCA, UV, ductus venosus (DV), inferior vena cava, descending aorta, and renal arteries for fetal assessment. Although evidence for improved outcome is still lacking, some centers are using several of these surveillance modalities in conjunction with ultrasound anatomic surveys and BPP to guide management of preterm growth restricted fetuses.
5. UA Doppler ultrasound velocimetry is the most commonly used Doppler surveillance for high-risk pregnancies, particularly those at risk for uteroplacental insufficiency.15 It is also used to distinguish between the growth restricted and constitutionally small fetus.
6. In the progression of placental dysfunction and FGR, once a third of the placental vasculature is affected by disease, UA S/D ratio is consistently increased, while after half of the fetal villi are obliterated, UA blood flow becomes pulsatile (exhibits absent or reversed end diastolic flow).13,36 Reversed end diastolic flow predicts increased rates of NICU admission and perinatal mortality,37–39 as well as long-term neurologic complications.40
7. There is a predictable progression of fetal responses to placental vascular dysfunction.
a. Before UA Doppler becomes abnormal, UV and DV flows are decreased—increased DV shunting away from the liver and associated alterations in glucose–insulin metabolism lead to decreased fetal abdominal circumference, observable before estimated fetal weight falls below the 10th percentile.
b. Decreased UA Doppler index ensues, and chronic deprivation of nutrients and oxygen result in subclinical delays in FHR milestones (higher baseline, lower variability, delays in achieving reactivity).
c. Increased MCA S/D reflects compensatory fetal central nervous system (CNS) autoregulation-induced increases in cerebral perfusion. Once reduced or absent EDV is observed, the BPP becomes abnormal within 2 to 3 days.
d. Biophysical parameters are affected sequentially in order of relative sensitivity to progressive acidosis.
(1) Decreased FHR variability
(2) Decreased fetal breathing
(3) Reduced amniotic fluid volume
(4) Finally, reduced fetal movement and tone
CLINICAL PEARLAbsent and reversed diastolic flow velocity is associated with fetal hypoxemia and acidosis and has been correlated with increased perinatal mortality.41–43
8. In a meta-analysis of 18 randomized and nonrandomized trials involving more than 10,000 high-risk pregnancies, use of Doppler ultrasound yielded a 29% reduction in perinatal mortality.44
9. In contrast, a systematic review of trials of UA Doppler as a screening test in low-risk pregnancies, including five studies with more than 14,000 women, failed to demonstrate any benefit.45
10. The decision to deliver hinges balancing fetal well-being versus delivery at an early gestational age.
a. Management of early-onset FGR (<34 weeks) emphasizes safe prolongation of pregnancy (preventing morbidity and mortality owing to preterm delivery).
b. Management of late-onset FGR emphasizes accurate diagnosis to prevent stillbirth.
V. Intrapartum fetal surveillance
A. Physiology
1. Uteroplacental perfusion is intermittently reduced by uterine contractions, resulting in repetitive hypoxia during labor.14
2. Normal maternal, uteroplacental, and fetal physiology confer a wide margin of safety for fetal oxygenation such that reduced oxygen delivery to the fetal circulation during labor contractions is usually well tolerated.
3. Neonates born after experiencing labor commonly exhibit mild cord blood acid–base abnormalities (mild respiratory acidosis with normal base deficit) compared to those born without experiencing labor, with no ill effect.
4. Contractions in the presence of greater degrees of uteroplacental insufficiency cause increasingly severe gas exchange impairment, causing fetal asphyxia and metabolic acidosis. If this is severe, it may contribute to neonatal hypoxemic ischemic encephalopathy.
5. The goal of intrapartum monitoring is to detect potential fetal hypoxemic/acid–base decompensation to allow for early intervention, first to improve gas exchange, then to deliver the baby, if necessary.
B. Intrapartum electronic fetal monitoring—nomenclature and interpretation
1. As noted earlier, despite demonstrating no evidence of long-term beneficial outcomes, intrapartum EFM is routinely performed, with the premise that EFM changes during labor will appear before potential neurologic injury to the fetus, allowing for timely intervention and hence mitigating neurologic injury.
2. The recommendations of the 2008 consensus workshop, sponsored by National Institute of Child Health and Human Development (NICHD), ACOG, and the Society for Maternal-Fetal Medicine, regarding EFM nomenclature and classification scheme and standard intrapartum management, have been adopted widely (ACOG; Association of Women’s Health, Obstetric and Neonatal Nurses; and American College of Nurse Midwives) and are as follows.2,18,46
a. Uterine activity. Interpretation of EFM occurs in relation to uterine activity, which is characterized as:
(1) Normal: five or fewer contractions per 10 minutes, averaged over a 30-minute window
(2) Tachysystole (formerly “uterine hyperstimulation”): more than five contractions in 10 minutes, averaged over 30 minutes.
b. Characterization of FHR includes five individual elements: baseline heart rate, variability, accelerations, decelerations, and changes in patterns or trends during labor, as follows:
(1) Baseline FHR is determined as the mean FHR rounded to 5 bpm during a 10-minute period, that is devoid of accelerations, decelerations, or marked variability.
(a) Normal: 110 to 160 bpm
(b) Tachycardia: greater than 160 bpm. Tachycardia may be caused by maternal fever, maternal medication administration, fetal cardiac dysrhythmias, or fetal asphyxia.
(c) Bradycardia: less than 110 bpm. Fetal bradycardia can occur as a consequence of fetal cardiac conduction defect, maternal medications, uteroplacental insufficiency, maternal hypothermia, or as a normal variant.
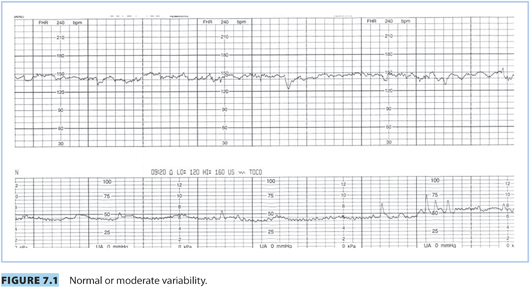
(2) Baseline variability is defined as the fluctuation in FHR and is visually represented by the amplitude of each wave, from trough to peak, in the FHR tracing. Accurate determination of baseline variability is made during a 10-minute window that is devoid of accelerations or decelerations.
(a) Normal or moderate variability: amplitude range of 6 to 25 bpm (Fig. 7.1)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







