Extubation: Introduction
It is easy to merge decisions about extubation with decisions about weaning in everyday practice. Indeed, much patient mismanagement is caused by conflating these two subjects. But the conflation is not confined to clinicians. Many researchers have also merged the two subjects, such as using weaning predictors to predict reintubation in a patient who has already tolerated a weaning trial. The result is scientific confusion.
When a patient tolerates a weaning trial without distress, a clinician feels reasonably confident that the patient will be able to sustain spontaneous ventilation after extubation. But this is not the only consideration. The clinician also has to consider whether the patient will be able to maintain a patent upper airway after extubation.
Removal of an endotracheal tube is typically performed under controlled conditions. The patient has satisfactorily tolerated a weaning trial. Enteral feeding is temporally withheld for approximately 4 hours. The patient is usually positioned in a sitting posture. The endotracheal tube, mouth, and upper airway are suctioned, paying attention to the collection of secretions above an inflated cuff. Some clinicians recommend keeping a suction catheter in place (aiming for the catheter to barely protrude from the distal end of the endotracheal tube) as the cuff is deflated; this step is taken in an attempt to capture any secretions sitting on top of an inflated cuff, which might fall into the airway after deflating the cuff. Some clinicians forcefully inflate the lungs with an Ambu Bag immediately before pulling out the endotracheal tube, hoping that the larger than usual ensuing exhalation will push secretions upward and outward. After removal of the endotracheal tube, the patient is given supplemental oxygen, titrated to oxygen saturation (SO2), being particularly cautious with a patient who is at risk of carbon dioxide retention. Patients may have impaired airway protection reflexes immediately after extubation. If speech is impaired for more than 24 hours, indirect laryngoscopy should be undertaken to assess vocal cord function. Oral intake should be delayed in patients who have been intubated for a prolonged period.
Postextubation Distress
Between 2%1,2 and 30%3–6 of patients experience respiratory distress in the postextubation period (Table 59-1). Many, but not all, require reinsertion of the endotracheal tube and mechanical ventilation. These patients are commonly classified as extubation failures, a term popularized by Demling et al.7 These investigators defined extubation failure as the need for reintubation within 7 days. Unfortunately, the meaning of extubation failure varies among authors, leading to scientific confusion. Even when authors employ it as a synonym for reintubation, the period under study varies—within 24, 48, or 72 hours, or as long as 7 days.
| Authors | Number of Patients | Percent Reintubated | Percent Mortality | Time Frame |
|---|---|---|---|---|
| Tahvanainen et al73 | 47 | 19.1 | 22.2 | — |
| DeHaven et al74 | 48 | 6.3 | NR | — |
| Demling et al7 | 400 | 4.4 | 40 | 7 days |
| Demling et al7 | 299 | 3.3 | 10 | 7 days |
| Krieger et al75 | 269 | 10.4 | NR | — |
| Mohsenifar et al76 | 29 | 14.3 | NR | 24 hours |
| Sassoon et al77 | 40 | 12.5 | NR | 48 hours |
| Brochard et al78 | 109 | 11 | NR | 48 hours |
| Lee et al79 | 52 | 17 | 33.3 | NR |
| Capdevila et al80 | 67 | 17.9 | NR | 48 hours |
| Esteban et al81 | 530 | 15.6 | NR | 48 hours |
| Torres et al29 | 170 | 23.5 | 35 | — |
| Chatila et al82 | 100 | 9.5 | <24 hours | |
| Dojat et al3 | 38 | 29.4 | 40 | 48 hours |
| Ely et al83 | 300 | 3.7 | NR | 48 hours |
| Leitch et al1 | 163 | 1.8 | NR | <24 hours |
| Miller et al35 | 88 | 17 | NR | NR |
| Epstein et al12 | 289 | 14.5 | 42.5 | — |
| Esteban et al28 | 484 | 18.6 | 27 | 48 hours |
| Jacob et al84 | 183 | 4.5 | NR | 24 hours |
| Kollef et al85 | 357 | 11.5 | NR | NR |
| Vallverdu et al86 | 217 | 15.5 | NR | 48 hours |
| Esteban et al15 | 526 | 13.5 | 32.8 | 48 hours |
| Zeggwagh et al33 | 101 | 37 | NR | 48 hours |
| Coplin et al45 | 136 | 17.6 | NR | NR |
| Koh et al87 | 36 | 19 | NR | 48 hours |
| Maldonado et al5 | 24 | 26.7 | NR | 24 hours |
| Khamiees et al43 | 91 | 12.8 | NR | 72 hours |
| Namen et al13 | 100 | 16 | NR | NR |
| Cohen et al4 | 35 | 28.6 | 10 | 48 hours |
| De Bast et al22 | 76 | 18.4 | NR | 24 hours |
| Perren et al88 | 98 | 6.7 | 33 | 48 hours |
| Smina et al16 | 95 | 11.3 | — | 72 hours |
| Conti et al2 | 92 | 1.7 | NR | 48 hours |
| Fernandez et al89 | 130 | 18 | NR | 48 hours |
| Francois et al21 | 343 | 8 | 0.3 | 24 hours |
| Thille et al31 | 168 | 15.5 | 50% | 72 hours |
Extubation failure is most often defined as the need for reintubation. The corollary of this definition is that all patients who do not require reintubation should be classified as extubation successes no matter how much difficulty they experience. A number of patients develop stridor after extubation, which resolves with inhalation of racemic epinephrine or other therapy without requiring reintubation. If extubation failure is defined as reintubation, such patients should be excluded. But if researchers are investigating the development of respiratory distress after extubation, all patients with significant postextubation stridor should be included. Much confusion could be avoided if researchers used the term reintubation when that is their sole criterion for extubation failure. If researchers use the term extubation failure, it seems logical to assume that their study population includes some patients with postextubation distress who did not require reintubation. In many reports, however, researchers do not make it clear which approach they are adopting—whether (or not) their category of extubation failure included some patients who developed distress after extubation but who did not require reintubation.
Investigators have variably classified patients who required noninvasive ventilation (NIV) after extubation as satisfying or not satisfying the definition of extubation failure. De Lassence et al8 specified that they excluded patients who were managed by NIV in a cohort of extubation failure patients. Maldonado et al5 included patients managed by NIV among their group of extubation failures, as did Haberthur et al9 and Jiang et al.10 Moreover, Haberthur et al9 extended the term extubation failure to include patients experiencing unjustified delay in extubation with one particular weaning technique if that patient tolerated extubation after being switched to an alternative weaning technique.
If patients die from a cardiorespiratory cause without being reintubated, it seems unscientific to classify them as extubation successes. Some authors11 have specified that their definition of extubation failure included both the need for reintubation or unexpected death within 72 hours. Most authors, however, do not address this issue. Some extubated patients refuse reintubation (as part of a decision for withdrawal of life support) and die. How should these patients be classified? Demling et al7 classified fatal outcomes as extubation failures, Epstein et al12 excluded such patients from their group of extubation failures, and Namen et al13 classified such patients (who died) as extubation successes.
How should patients who experience unplanned extubation followed by reintubation be classified? In a group of forty-two extubation failures, Epstein et al12 included four unplanned extubations (who required reintubation within 72 hours) because these occurred while a weaning trial was in progress, but excluded sixteen other unplanned extubations (requiring immediate extubation) because these did not occur during weaning trials. If patients are extubated because of a defective endotracheal tube and then reintubated, how should they be classified? Epstein and Ciubotaru14 excluded such patients, but many authors do not state clearly how such patients are classified.
Some patients may be inappropriately reintubated because of poor clinical judgment. What criteria should be set for judging a reintubation as appropriate? Epstein and Ciubotaru14 listed the following criteria: increase in arterial carbon dioxide tension (PCO2) greater than 10 mm Hg and decrease in pH of 0.10; arterial oxygen tension (PO2) less than 60 mm Hg or less than 90% with an inspired oxygen concentration (FIO2) greater than 0.50; signs of increased work of breathing (high respiratory rate, accessory muscle use, or paradoxical breathing); and inability to protect the airway (secondary to upper airway obstruction or excess secretions). Many of these criteria are similar to those used for defining weaning failure, but it is more difficult to ensure their rigorous and consistent application as criteria for reintubation. Weaning failure typically occurs under controlled conditions, usually within 1 hour of starting a weaning trial. Reintubation for postextubation distress may not occur until many hours after extubation, and the listing of satisfied criteria may not be entered on a data form until hours after the event.
Causes and Pathophysiology of Postextubation Distress
The listed indications for reintubation vary considerably from study to study. Of these, postextubation stridor has attracted the most attention.
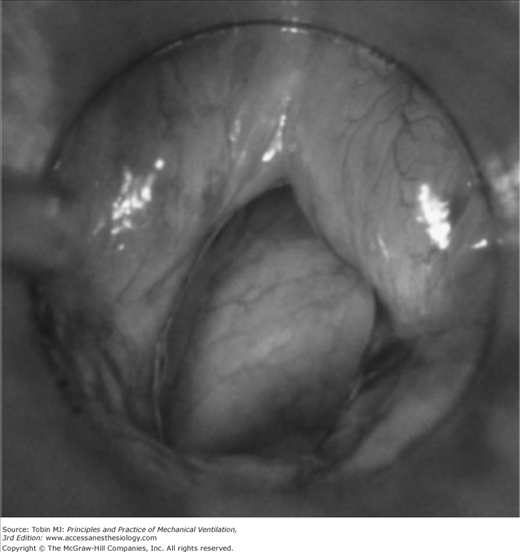
A number of investigators have reported that upper airway obstruction accounts for approximately 15% of patients requiring reintubation (15% in the study of Epstein and Ciubotaru;14 14.7% in that of Esteban et al;15 and 15.4% in that of Smina et al16). These investigators, however, did not report what proportion of patients who developed clinical manifestations of upper airway obstruction did not require reintubation. Upper airway obstruction may result from edema of the subglottic area or the vocal cords, reflex closure of the vocal cords (laryngospasm), or compromise of the tracheal lumen (tracheomalacia or compression by a hematoma) (Figs. 59-1 and 59-2). The laryngeal edema that occurs in intubated patients is believed to arise from direct mechanical trauma to the larynx by the endotracheal tube. Almost every patient intubated for 4 days or more develops laryngeal edema and mucosal ulcerations, usually located posterior to the level of the vocal cords, where the tube exerts the highest pressure.17 Laryngeal edema is usually transient and self-limiting, and most of the lesions resolve within 1 month.18
Figure 59-1
Mild postextubation laryngeal edema as seen with a rigid laryngoscope. (Used, with permission, from Antonaglia et al.102)
Figure 59-2
Moderately severe postextubation laryngeal edema as seen with a rigid laryngoscope. (Used, with permission, from Antonaglia et al.102)
Upper airway obstruction causes stridor only if the patient is capable of generating sufficient airflow; if airflow is insufficient, obstruction may cause hypercapnia, hypoxemia, or paradoxical breathing. Of 110 extubated patients, Sandhu et al19 observed that thirteen (11.8%) developed stridor, but less than half of the patients with stridor (6/13) required reintubation (no patient required reintubation for any reason other than stridor in this series). Jaber et al20 observed stridor in thirteen of 112 (11.6%) extubated patients, and nine of the thirteen required reintubation (only 2% [2/99] of patients without stridor required reintubation). Francois et al21 undertook a randomized controlled trial of the effect of steroids on postextubation laryngeal edema in 761 critically ill patients requiring at least 36 hours of mechanical ventilation. Of the 343 patients in the placebo arm, seventy-six (22%) exhibited features of postextubation laryngeal edema. Twenty-six of the 343 extubated patients (8%) required reintubation, and the reintubation was linked to laryngeal edema in fourteen (54%[14/26]) of these patients. Forty-six percent of the patients with postextubation stridor or endoscopic laryngeal edema did not require reintubation, indicating that the development of stridor is not a particularly strong predictor of the need for reintubation.17
In the above studies, upper airway obstruction was typically diagnosed on the basis of clinical manifestations. De Bast et al22 undertook fiber-optic examination before reintubation or directly inspected the glottis during reintubation to confirm the presence of laryngeal edema. Of seventy-six patients who had been intubated for at least 12 hours, fourteen required reintubation within 24 hours of extubation (reintubation rate, 18.4%). Of these fourteen patients, eight (57.1%) had laryngeal edema.
When upper airway obstruction occurs, it is typically manifested soon after extubation. Of the seventy-six patients who developed features of laryngeal edema in the study of Francois et al,21 47% (36/76) developed it within 5 minutes of extubation, 34% (26/76) developed it between 6 and 30 minutes after extubation, and 18% (14/76) developed it 30 minutes or longer after extubation.
Although laryngeal edema can develop as early as 6 hours after intubation,22 many, but not all,23 investigators have noted that the rate of postextubation stridor increases in proportion to the duration of ventilation. Jaber et al20 observed that duration of intubation was longer in thirteen patients with stridor than in ninety-nine patients without stridor: 10.9 versus 5.5 days. Sandhu et al19 likewise observed a longer duration of intubation in six patients who developed postextubation stridor than in ninety-seven patients who did not: 6.5 ± 1.9 versus 2.6 ± 2.6 days. Darmon et al24 observed that stridor was more common among patients intubated for longer than 36 hours than among patients intubated for a shorter time: 7.2% (25/346) versus 0.9% (3/317).
Women are more susceptible to postextubation stridor than men, and the rate may vary with ethnicity. In a series from France, Darmon et al24 observed stridor in 7.4% (20/284) of women and 2.1% (8/379) of men. In a series from Taiwan, Ho et al23 observed stridor in 39% (7/18) of women and 17% (10/59) of men.
Other risk factors associated with the development of laryngeal edema include traumatic intubation, excessive tube size, excessive tube mobility secondary to insufficient fixation, a patient fighting against the tube or trying to speak, excessive pressure in the cuff, too frequent or too aggressive tracheal suctioning, occurrence of infections or hypotension, and the presence of a nasogastric tube that predisposes to gastroesophageal reflux.22 It is also possible that a biochemical reaction between the tube material and the airway mucosa may cause laryngeal edema.22 Compared with the ninety-nine patients without stridor, the thirteen patients who developed stridor in the series of Jaber et al20 were more likely to have the following: a traumatic and/or difficult intubation (54% vs. 7%), a history of self-extubation (38% vs. 4%), a higher balloon cuff pressure (83 vs. 40 cm H2O), a higher simplified acute physiology score (SAPS) II score (50 vs. 38), and a medical rather than a surgical reason for admission (46% vs. 18%).
Conditions other than upper airway obstruction that cause postextubation distress vary from study to study. In a report on reasons for reintubation, Epstein and Ciubotaru14 noted upper airway obstruction in 15%; other reasons for reintubation included respiratory failure (28%), congestive heart failure (23%), aspiration or excessive secretions (16%), encephalopathy (9%), and other conditions (8%). The frequency of a particular reason differs among studies. For example, cardiac failure accounted for 23% of the cases of Epstein and Ciubotaru14 and 6.6% (4/61) of the cases of Esteban et al,15 but none of the cases of Smina et al16 or De Bast et al.22 Because of the limited rigor of these studies, there is little point in attempting a more detailed analysis of the relative incidence of other causes of reintubation.
None of the studies of reasons for postextubation cardiorespiratory distress can be considered as studies of pathophysiologic mechanisms in the same sense as are studies of the pathophysiology of weaning failure. For studies of postextubation distress, investigators filled out case report forms. These forms constitute post hoc incident reports completed after some event. In many cases, investigators are making a best guess as to what might explain a patient’s deterioration. In contrast, research into the pathophysiology of weaning failure is based on the simultaneous recording of several signals, starting before a weaning trial and continuing until after its completion. In this way, it is possible to understand the relative roles of control of breathing, respiratory muscle activity, derangements of lung and chest wall mechanics, gas exchange, and cardiovascular performance in weaning failure. Conducting similar types of studies to delineate the mechanisms of postextubation cardiorespiratory distress will be challenging.
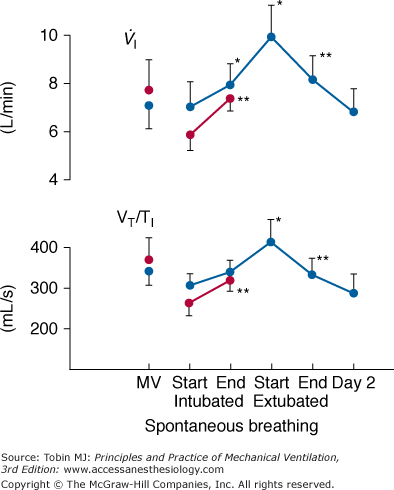
The first challenge is instrumentation. The recording of swings in intrathoracic pressure, as reflected by esophageal pressure, is relatively easy. But on its own, esophageal pressure is of limited value. Derivation of most indices, such as airway resistance, compliance, and intrinsic positive end-expiratory pressure, require a simultaneous measurement of airflow. It is extremely difficult to obtain a meaningful measure of airflow or tidal volume in a recently extubated patient.25 The use of a mouthpiece or face mask causes marked distortion of the breathing pattern. Inductive plethysmography provides a means for overcoming this problem. For example, Tobin et al26 used this technique to study changes in breathing pattern in ten patients at the point of extubation (Fig. 59-3). During the first 15 minutes after extubation, both minute ventilation and mean inspiratory flow (a measure of respiratory motor output) increased, accompanied by a decrease in the degree of abdominal paradox. By the end of the first hour after extubation, respiratory drive and minute ventilation had returned to preextubation levels. No further change in breathing pattern was observed over the subsequent 24 hours. The investigators did not study any patients who developed postextubation distress. When inductive plethysmography is combined with esophageal pressure recordings, great care is required to ensure that the two signals are perfectly aligned. The smallest misalignment will cause major errors in estimates of intrinsic positive end-expiratory pressure and other measures of lung mechanics. A requirement not faced by researchers studying the pathophysiology of weaning failure is the need to record the development of laryngeal obstruction. As such, additional research instrumentation includes fiber-optic endoscopy.
Figure 59-3
Recordings of minute ventilation ( ) and mean inspiratory flow (VT/TI), a measure of respiratory motor output, during mechanical ventilation (MV), at the start and end of a T-tube trial, the first 15 minutes after extubation, 45 to 60 minutes after extubation, and 24 hours after extubation. Ten patients who tolerated the T-tube trial and were extubated are shown in blue and seven patients who failed the T-tube trial and were reconnected to the ventilator are shown in red. Bars represent ±1 standard error (SE); *, p < 0.05; **, p <.01 compared with the value during the immediately preceding time-block of spontaneous breathing. (Used, with permission, from Tobin et al.26)
) and mean inspiratory flow (VT/TI), a measure of respiratory motor output, during mechanical ventilation (MV), at the start and end of a T-tube trial, the first 15 minutes after extubation, 45 to 60 minutes after extubation, and 24 hours after extubation. Ten patients who tolerated the T-tube trial and were extubated are shown in blue and seven patients who failed the T-tube trial and were reconnected to the ventilator are shown in red. Bars represent ±1 standard error (SE); *, p < 0.05; **, p <.01 compared with the value during the immediately preceding time-block of spontaneous breathing. (Used, with permission, from Tobin et al.26)
Perhaps an even greater challenge than the instrumentation is the timing. Weaning failure almost invariably occurs within the first hour of attempted spontaneous breathing. The time course for the development of postextubation cardiorespiratory distress extends over a longer span. In the study of Epstein and Ciubotaru,14 for example, only 33% of reintubations occurred within the first 12 hours after extubation, and 42% occurred after 24 hours.
Consequences of Postextubation Distress
Many, but not all,27 investigators have reported that mortality is many times higher in patients who require reintubation than in patients who tolerate extubation (Table 59-2). Three explanations have been offered to account for the increased mortality: complications associated with the act of reintubation itself; development of a new problem in the interval between extubation and reintubation; and the need for reintubation is simply serving as a marker for a poor prognosis.
Endotracheal intubation is typically performed under elective and controlled conditions. It is more challenging to perform intubation in a patient developing acute distress in the period after extubation. Complications have been reported to occur at the time of reintubation in 15%,28 18%,15 and 28% of patients.14 In a study of forty consecutive patients requiring reintubation (for any reason), Torres et al29 reported that 47% (19/40) developed nosocomial pneumonia after reintubation, as compared with 10% of matched control patients (odds ratio [OR]: 5.9). In a study of 297 intubations performed under emergency conditions (in 238 adults), Schwartz et al30 reported seven deaths (mortality: 2.4%) at the time of or within 30 minutes after intubation; five of the deaths were associated with a systolic blood pressure less than 90 mm Hg. In contrast to the experience of Torres et al,29 only 4% of patients developed a radiographic infiltrate compatible with a new aspiration pneumonia.30 In a study by Esteban et al,15 mortality was no greater among the eleven patients who developed complications at the time of reintubation than in the remaining fifty patients (45.4% and 30.0%, respectively; p = 0.53). Based on the above considerations, it seems unlikely that the higher mortality in reintubated patients is a direct consequence of complications associated with the act of reintubation itself.
A second explanation is the development of a new problem during the interval between extubation and reintubation. In support of this possibility is the observation of Epstein and Cibotaru14 that mortality increases in proportion to the time between extubation and reintubation: mortality of 69% in patients reintubated between 49 and 72 hours after extubation (17% of the group), 24% in patients reintubated in the first 12 hours after extubation (33% of the group), and 39% in patients reintubated between 13 and 24 hours after extubation (25% of the group).
The third explanation for higher mortality in reintubated patients is that reintubation is simply serving as a marker for a poor prognosis. Sicker patients are more likely to undergo reintubation. Epstein and Ciubotaru14 have argued against this explanation. They note that reintubation continues to have a strong independent effect on mortality even after controlling for generalized severity of illness at weaning onset, comorbidity, age, and need for acute dialysis. It is, however, possible that the need for reintubation is measuring some additional aspect of disease severity not captured by the above variables.
Thille et al31 have argued that reintubation has a direct and specific effect on patient outcome because it is frequently followed by marked clinical deterioration. They studied twenty-six patients who failed extubation and required reintubation within the subsequent 72 hours; 50% of the reintubated patients died in the ICU. At the time of extubation, the Sequential Organ Function Assessment (SOFA) score was not significantly different between the twenty-six patients who subsequently required reintubation and 142 patients who tolerated extubation. In the first 24 hours after extubation, the SOFA score increased significantly from 3.4 ± 2.9 to 4.7 ± 3.4 in the failed extubation group, mainly as a result of hemodynamic and respiratory deterioration. Patients who tolerated extubation showed an improvement in their SOFA score over the same interval, essentially because of removal of the ventilator. These data suggest that reintubation leads to adverse consequences, and it is not simply a marker for a poor prognosis.
Predictors of Postextubation Distress
Because reintubation causes serious complications in some patients, attempts are made to predict its likely occurrence. A number of physiologic variables have been evaluated for their ability to predict this likelihood. For some patients, the likelihood of reintubation is considered so high that a clinician may proceed to tracheotomy without first attempting extubation.
It is extremely uncommon to undertake planned extubation without first assessing a patient’s ability to sustain spontaneous ventilation. This assessment typically consists of observing a patient breathing through a T-tube circuit or while assisted by a low level of pressure support or intermittent mandatory ventilation. A weaning trial serves primarily as an additional diagnostic test, with the aim of predicting whether a patient will develop distress after extubation and need reintubation. The predictive accuracy of a weaning trial as a diagnostic test has never been evaluated in a rigorous scientific manner.
A true-positive result of a T-tube trial is defined as a patient who tolerates the trial without distress, is then extubated, and does not require reintubation. The usual rate of reintubation is 15% to 20% (sometimes lower), but higher reintubation rates have been reported by some investigators: 23.5%,29 25%,32 26.7%,5 28.6%,4 and 29.4%.3 These false-positive test results mean that the positive-predictive value and specificity of passing a T-tube trial in predicting that a patient will not require reintubation is much less than 100%. To measure the false-negative rate would require extubating patients who fail a T-tube trial, and counting how many do not require reintubation. For obvious ethical reasons, we do not know this number. Given the natural caution of physicians, we can confidently assume that it is higher than 0%. As such, sensitivity and negative-predictive value will be less than 100%. It is no surprise that the ability of a T-tube trial to predict reintubation has a sensitivity and specificity of less than 100%; no diagnostic test is perfect. But a weaning trial is not solely used as a diagnostic test for predicting the likelihood of reintubation. The outcome of a weaning trial is also used as a reference standard against which the accuracy of weaning predictor tests are measured.
Several investigators have investigated the ability of weaning predictor tests to predict the development of distress after extubation. The question posed is along these lines: “Does frequency-to-tidal-volume ratio (f/VT), or some other predictor test, measured before a T-tube trial, predict the likelihood of reintubation?” To answer this question with scientific validity, it is imperative that the investigators take clearly defined steps to ensure that clinicians are not taking the results of the T-tube trial into account when deciding whether to extubate the study patients. (In other words, a decision to extubate the patient must be taken before the T-tube trial, and must proceed even if the patient exhibits significant distress during the trial.) If researchers allow clinicians to use results of a T-tube trial (done after measurement of the weaning predictor test) when deciding whether or not to proceed with extubation, the researchers need a different experimental design because they are asking a different research question. The question is now, “In what instances do weaning predictor tests override the results of a subsequently undertaken T-tube trial?”
Before we discuss the findings of studies on the use of tests to predict the likelihood of postextubation distress, we ask the reader to undertake a simple thought experiment. You, as the patient’s clinician, record f/VT, and obtain a reading of 60. You then proceed to a T-tube trial. If the patient develops severe distress during the trial, would you extubate the patient? (Please exclude circumstances in which you believe that the internal diameter of the endotracheal tube is the main cause of distress.) We believe that most experienced clinicians will answer“no.” Consider another scenario: A resident measures f/VT in your patient, obtains a value of 120, and proceeds to a T-tube trial. If the patient tolerates the trial without significant distress, would you defer extubation? We believe most experienced clinicians would again answer “no,” although they might monitor the postextubation period more closely than if the patient had an f/VT reading of 90 before the trial.
For both of the preceding scenarios, we believe that few if any experienced clinicians would allow a measurement of f/VT (made before a T-tube trial) to override a judgment based on how well a patient tolerates a T-tube trial. Given the results of the thought experiments, it makes little sense to undertake research studies of the accuracy of weaning predictor tests (measured before a weaning trial) to forecast a patient’s likely need for reintubation (in a patient who passes a weaning trial). It makes even less sense when one considers that a clinician’s action based on the patient’s performance during the weaning trial will have inevitably muddied the experimental waters. Let us consider a patient in whom a weaning predictor test predicts a high likelihood of respiratory distress. A weaning trial is nevertheless undertaken. The patient develops distress, and so is not extubated. By excluding such patients from a study, the investigators are markedly underestimating the true-negative rate of the test for predicting distress after extubation (where the test predicts distress and the patient actually develops distress).
It is difficult to understand why so many investigators have undertaken this type of research. We suspect they have been seduced by affirmative answers to two subsidiary questions. “Do patients with satisfactory weaning predictors usually tolerate a spontaneous breathing trial?” Yes. “Do patients who tolerate a spontaneous breathing trial usually avoid reintubation?” Yes. It might seem logical to conflate these two issues, and ask:“Do patients with satisfactory weaning predictors usually avoid reintubation?” The only way to address this question in a scientific manner is to measure weaning predictors and extubate the patient without an intervening weaning trial. Zeggwagh et al33 are the only group of investigators to undertake such a study.
The investigators prospectively studied 101 patients (ventilated for 10.4 ± 10.3 days) at the point that their ICU physicians contemplated weaning. They measured a series of physiologic measurements during 2 minutes of spontaneous breathing; the results of these measurements were not communicated to the primary team. The team then extubated the patients without first undertaking any form of weaning trial. The extubation decision was made by the ICU team, based on the following criteria: improvement or resolution of the condition precipitating the need for mechanical ventilation; good level of consciousness with cessation of all sedative agents; temperature less than 38°C (100.4°F); respiratory frequency less than 35 breaths/min; SO2 greater than 90% on FIO2 equal to or less than 0.40; hemodynamic stability; and the absence of electrolyte disorders, acid–base disturbance, or hemoglobin less than 10 g/dL. Reintubation was necessary in 37% of the patients. Several variables predicted the need for reintubation with a reasonable degree of accuracy. For example, f/VT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree