Decreased compartmental volume
•Excessive traction with fracture immobilization
•Closure of fascial defects following trauma
Increased compartmental content
•Intracompartmental bleeding secondary to fractures, vascular injury, or bleeding disorders
•Increased capillary filtration following reperfusion after ischemia, embolectomy, soft tissue trauma, burns, or fracture fixation
Externally applied pressure
•Tight immobilization of fractures
•Lying on limb
Clinical Presentation
In awake, alert patients, most extremity compartment syndromes can be diagnosed by clinical examination alone. The common clinical signs include the five Ps: pain, pulselessness, pallor, paresthesias, and paralysis. Additional Ps to consider include pressure (swelling and tenseness of the compartment), and poikilothermy (Fig. 38.1). Other signs to look for are skin edema, blisters, swelling, and subcutaneous blood suffusions (Fig. 38.2).
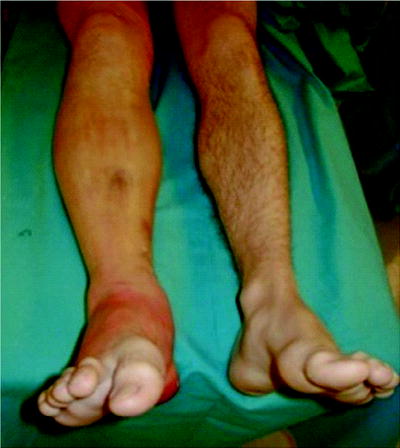
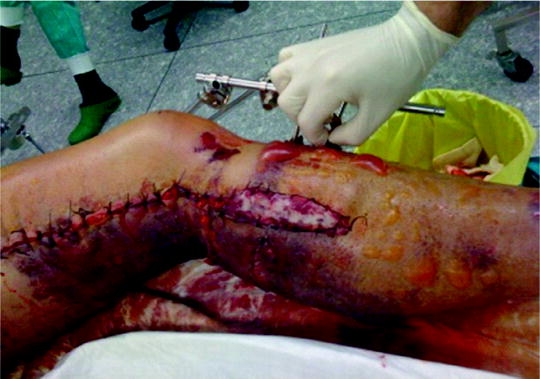

Fig. 38.1
A case of right lower extremity compartment syndrome secondary to an isolated tibial fracture. Pain, swelling, and the inability of dorsal flexion of the toe was noted on examination

Fig. 38.2
A case of left lower left extremity compartment syndrome following a popliteal artery injury and subsequent revascularization in combination with a proximal tibial fracture externally fixed. Several hours following this management, there are obvious signs of compartment syndrome to include swelling, skin discoloration and blistering, and functional loss
To make a diagnosis, a physician must first have an evidence of increased intracompartmental pressure. If so, the signs do not occur simultaneously, but they develop with time. One of the first signs is a swollen or tight compartment in combination with severe pain that is out of proportion of what it is expected to be and which is not relieved by regular analgesia. Other signs are late presentation and often, when this is present, an irreversible damage to soft tissues has occurred. There are numerous other pathophysiological events that can cause a similar clinical picture. In fact, a large meta-analysis of studies comparing clinical signs with development of acute lower extremity compartment syndrome showed sensitivity of 13–19%, specificity of 97%, positive predictive value of 11–15%, and negative predictive value of 98% [17]. Thus, the absence of this sign does rule out compartment syndrome, but the presence rarely confirms the correct diagnosis.
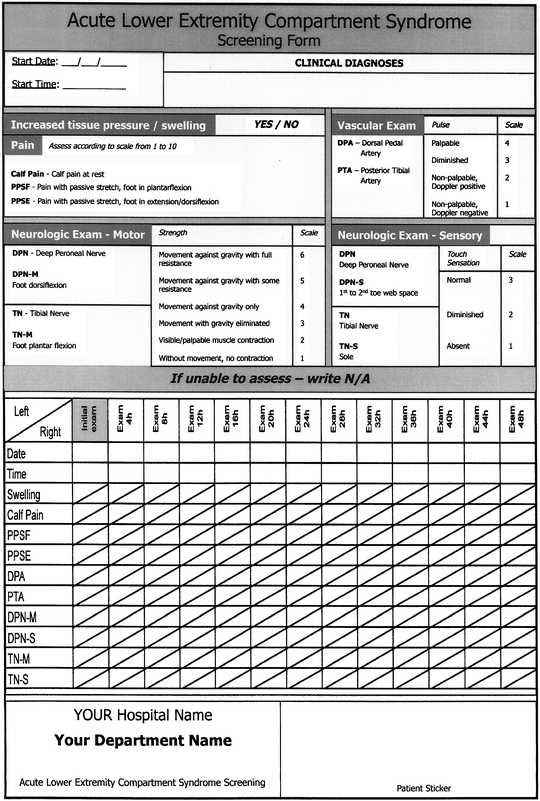
Nevertheless, clinical observation of the suspected compartment is what is recommended, since the hourly progress of the symptoms is what should be noted first before making a definitive diagnosis and starting definitive treatment. A useful tool for observation could be a simple sheet with notes about date, time, location, pain level, motor, and sensory testing. To do this, one must know the anatomical position of various compartments and their vascular and nerve content. Figure 38.3 shows an example of a screening form of the most commonly observed acute lower extremity compartment [18].
Diagnosis
To make a diagnosis of compartment syndrome we must have evidence of increased tissue pressure, inadequate tissue perfusion, and loss of tissue function. When all the three factors are present, the diagnosis may be made with assurance; when one or more of these factors are absent, the diagnosis is less secure. Evidence of increased tissue pressure may include complaints of tightness or pressure in the involved area. By palpation, the physician may perceive the tenseness of the compartmental envelope [19].
Evidence of inadequate perfusion of local tissue pressure may include the symptom of pain that is out of proportion to what would be anticipated from the clinical situation. Patients requiring increasing analgesic medication in a properly immobilized leg should raise suspicion. Pain on passive stretching of the intracompartmental muscles is another useful indication of increased pressure, especially if muscles have not been injured. Reduced peripheral pulses are very late signs of compartment syndrome; in fact, studies have shown normal pulses with Doppler signals in otherwise severely elevated intracompartmental pressures. Arterial flow is rarely compromised in elevated tissue compartment pressures. On the other hand, diminished pulses could be a result of other causes (e.g., vascular lesions) and in combination with reperfusion injury could also lead to development of compartment syndrome [19].
Evidence of abnormal tissue function includes weakness of the intracompartmental muscles and nerves, including sensory branches that cross-involve compartments and their lesion produces hypoesthesia. Both nerve and muscle function may be altered by direct injury; therefore, evidence of progressive loss of function over time may be a better sign [19].
To summarize, in awake and cooperative patients who can be reexamined frequently, the diagnosis of compartment syndrome is associated with the following findings:
1.
Pain that is out of proportion to what is anticipated from the clinical situation
2.
Weakness of the muscles in the compartment
3.
Pain on passive stretching of the muscles in the compartment
4.
Hypoesthesia in the distribution of the nerves coursing through the compartment
5.
Tenseness of the compartmental envelope
Since clinical signs are progressing and because they do not appear all at once, the clinical decision-making results in a conundrum [12, 20–22]. Especially in critically ill patients who are unable to cooperate because of head trauma, sedation, or even neuromuscular blocking drugs, the diagnosis cannot be made based on clinical examination alone [18].
Although the clinical examination should be a cornerstone of the diagnosis of compartment syndrome, it has disadvantages of being subjective and requires patient cooperation [12, 17, 23]. Therefore, tissue pressure measurement should be performed to add vital information for establishing the diagnosis and starting immediate treatment. The normal interstitial tissue pressure in the compartment is around 5 mmHg. Capillary blood flow becomes compromised at 20 mmHg, pain develops at pressures between 20 and 30 mmHg. A tissue pressure of more than 45 mmHg has been reported to be usually associated with compartment syndrome, and a pressure of more than 60 mmHg can confirm diagnosis [24–26]. But, the tolerance of tissue for increased pressure may be reduced by other factors, such as arterial occlusion, limb elevation, and shock [25, 27]; with these conditions compartment syndrome may occur at significantly lower interstitial pressures. According to arteriovenous gradient theory, the local blood flow (LBF) depends on the pressure gradient between arteries (P a) and veins (P v) and local vascular resistance to flow (R). This condition describes the formula [28]:
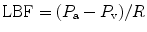
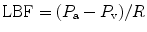
Local blood flow should be maintained to deliver enough oxygen to the tissues. According to this relationship, it is not the interstitial pressure that increases resistance, the only factor that reduces blood flow. The arterial pressure is also important, whereas venous pressure is somehow related to interstitial pressure. Increasing interstitial pressure also increases venous pressure, and furthermore decreases blood flow.
Because tolerance of tissues to increased intracompartmental pressure varies among different individuals and there are more factors that influence local blood flow, only one isolated measurement of interstitial pressure could not be enough to diagnose this condition. For example, higher compartment pressures may be necessary before injury occurs to peripheral nerves in patients with systemic hypertension [25], while compartment syndrome may develop at lower pressures in those with hypotension and/or peripheral vascular disease [29, 30]. It has been proposed that the difference between diastolic pressure and intracompartmental pressure is a better marker for compartment syndrome. A dP is calculated as follows:
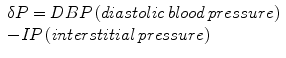
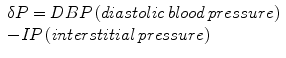
There are numerous methods of tissue pressure measurements [35–38]. The most commonly used are commercial handheld pressure monitors (e.g., Stryker™ device, Stryker, Kalamazoo, Michigan), a simple needle manometer system, and the wick or slit catheter technique. The question is accuracy, since there are reports that the arterial line manometer is the most accurate device [39]. The arterial line manometer device has another advantage of being able to monitor pressure continuously. Whichever method is used to measure compartment pressures, accuracy depends upon proper calibration of the measuring device and placement of the needle of pressure sensor in the level of the injured compartment. The principle of tissue pressure measurement in the case of acute lower extremity compartment syndrome is shown in Fig. 38.4.
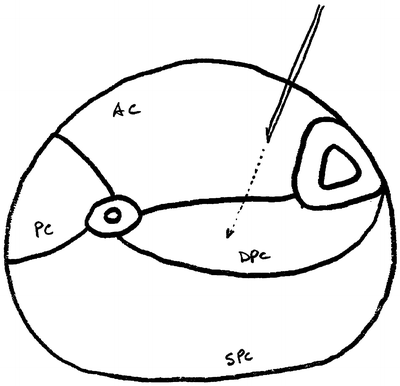

Fig. 38.4
An anatomical cross section of the lower leg and the location for needle placement in the anterior compartment (then proceeding into the deep posterior compartment) for pressure measurements. AC anterior compartment, PC peroneal (lateral) compartment, DPC deep posterior compartment, SPC superficial posterior compartment
Use of near-infrared spectroscopy for detection of low tissue oxygenation and therefore development of compartment syndrome is controversial. It has been reported as a useful noninvasive tool in diagnosing compartment syndrome after surgical revascularization of lower limb ischemia, whereas other studies did not prove its use due to severe edema of the soft tissues and inability to measure StO2 inside the muscle compartment [21, 33, 40–42].
In establishing a diagnosis of compartment syndrome we have to rule out other causes of pain-producing symptoms or we have to confirm a cause of elevated intracompartmental pressure. Especially with trauma, consider obtaining workup for rhabdomyolysis (creatinine phosphokinase (CPK), renal functions, urinalysis, and urine myoglobin). Extremity X-ray or CT scan can confirm the presence of fracture. MRI or ultrasonography can show muscle tears. Doppler ultrasonography or arteriography can detect vascular abnormality.
Management
The objective of treatment of a compartmental syndrome is to minimize deficits in muscular and neurological function by promptly restoring local blood flow. Certain nonoperative measures may be effective, such as eliminating external pressure and maintaining local arterial pressure. When there is external pressure that causes compartment syndrome, such as tight casts, it is essential to release the envelope immediately (remove and exchange for noncircular splint) when there is only one symptom or sign present. Usually this is pain and is most often observed in patients with fracture splints several hours after treatment. Restoration of normal limb perfusion has priority over closed fracture treatment and this is postponed until the perfusion returns to normal. Before using operative methods for reducing tissue pressure, we have to consider improvement of local blood flow if it has been reduced by shock, peripheral vascular disease, or elevation of the limb above the heart. All causes of systemic hypotension should be treated. Limb elevation should be avoided, because it lowers local arterial pressure and does not help to reduce swelling [43, 44]. Use of vasodilatating drugs or sympathetic blockade appears to be ineffective, because in this condition local maximal vasodilatation is already present. The use of phosphodiesterase inhibitor in experimental animals caused modulation of compartmental pressures. In a large study of trauma patients with isolated arterial injury, early anticoagulation with heparin has been found to reduce the incidence of compartment syndrome without significant bleeding as a consequence [45, 46].
The primary goal of treating compartment syndrome is to decrease intracompartmental pressures. Surgical decompression of all limiting envelopes is the gold standard of treatment indicated in the presence of a characteristic clinical picture of compartmental syndrome in a cooperative patient. When clinical exam is unreliable or difficult to obtain we have to consider pressure measurement, where either pressure should not exceed 45 mmHg or rather deltaP should not be below 30 mmHg.
The standard way of treatment is long skin incision and fasciotomy of all involved compartments and debridement of obvious nonviable tissue. Procedure should be performed without a tourniquet to avoid prolonging of ischemia and to permit the surgeon to assess degree of viability and restoration of blood flow. The skin is incised through the entire length of the involved compartment. There is obvious muscle bulging observed in a true compartment syndrome. Only obvious necrotic muscle should be removed, because tissue may have potential for reperfusion and recovery. The sign of contractility with electro stimulation should not be used initially. After fascial release we should anticipate post-ischemic swelling, and therefore, the skin should be left open and the wound temporarily closed with a patch of compliant artificial temporary skin closures. If release of the compartment is not complete, “rebound” compartment syndrome may occur.
After surgical decompression and temporary skin closure, sterile dressings are applied and the extremity is usually splinted in a functional position. In presence of fractures we have to consider fixation with external fixators, rarely with plates or intramedullary nails. This stabilization is performed immediately after fascial decompression and greatly facilitates later care of the wound, limb, and fracture. Passive stretching exercises are instituted to maintain range of joint motion. Skin closure may usually be accomplished 3–5 days after surgical decompression, usually by mesh-graft, rarely by direct suturing (Fig. 38.5). At that time, additional debridement of nonviable tissue can be performed. Fascial closure is not recommended, because this requires closure under tension and can lead to development of the compartment syndrome again. Muscle hernia is left behind and should be large enough not to cause additional late problems. When optimal cosmetic result is desired, one may progressively approximate the wound edges over 7–14 days with sutures to achieve direct skin closure.
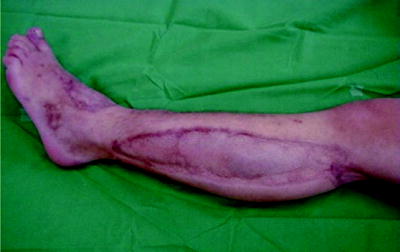

Fig. 38.5
Cosmetic result of a lower extremity compartment syndrome following split thickness skin grafting
Negative pressure wound care closure devices can be useful in the management of fasciotomy wounds. Negative pressure decreases wound edema, facilitates approximation of the skin edges, enhances local blood flow, promotes granulation tissue, and decreases bacterial colonization. There are no prospective randomized trials comparing standard sterile dressings compared to negative pressure treatment. But in retrospective analyses, negative pressure led to a significantly higher rate of complete skin closure and decreased time to skin closure [47, 48]. Hyperbaric oxygen as an adjunct to management following fasciotomy is reported in some case reports and animal studies, but there is lack of evidence to show an advantage compared to current practices [49–53].
Fasciotomy Techniques
The fasciotomy technique depends on the underlying condition or mechanism that caused compartment syndrome. The length of the lower extremity skin incisions have been debated for a long time. Minimal skin incisions with more extensive fascial incisions could place patient at risk for recurrent compartment syndrome [54–56]. The degree of muscle swelling after reperfusion cannot be predicted. Peak edema occurs several hours later after surgery.
Fasciotomies of the Upper Extremities
The upper extremity is anatomically divided into the brachium, antebrachium, and hand. Each of these anatomical segments has a different number of compartments with various muscle functions. The techniques for release of these compartments have to be discussed separately.
Fasciotomy of the Arm
The arm has two compartments: anterior, which includes the biceps and brachioradialis muscles, and posterior with the triceps muscle. Fasciotomy technique includes lateral skin incision from deltoid insertion to lateral epicondyle. Care must be taken to avoid damage to larger cutaneous nerves. At fascial level, intermuscular septum between anterior and posterior compartment is identified and fascia overlying each compartment is released with longitudinal incisions. The radial nerve should be protected as it passes through the intermuscular septum prom posterior compartment to the anterior compartment just below the fascia.
Fasciotomy of the Forearm
The antebrachium has three muscular compartments: mobile wad proximally, volar compartment, and dorsal compartment. Fasciotomy technique consists of a longitudinal centrally placed incision over the extensor compartment and a curvilinear incision on the flexor aspect beginning at the antecubital fossa (Fig. 38.6). A palmar incision is made between the thenar and hypothenar muscles in the palm, where the carpal tunnel can be released if needed. The incision is extended transversely across the wrist flexion crease to the ulnar side of the wrist, then arched across the volar forearm back to the ulnar side at the elbow. At the elbow, the incision is curved just radial to the medial epicondyle across the elbow flexion crease and the deep fascia is released. At the antecubital fossa, the fibrous band overlying the brachial artery and median nerve is carefully released. This incision allows for soft tissue coverage of the underlying neurovascular structures at the wrist and elbow and prevents soft tissue contractures from developing at flexion creases. A second straight dorsal incision can be made to release the mobile wad if necessary.
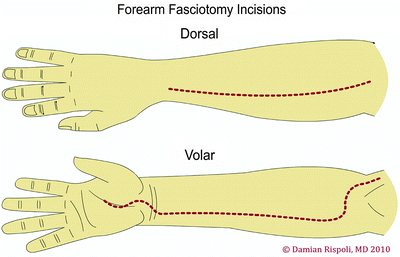

Fig. 38.6
Fasciotomy of the forearm—dorsal and volar aspects. Reprinted by permission of Data Trace Internet Publishing, LLC. Wheeless Textbook of Orthopaedics, Copyright 2011
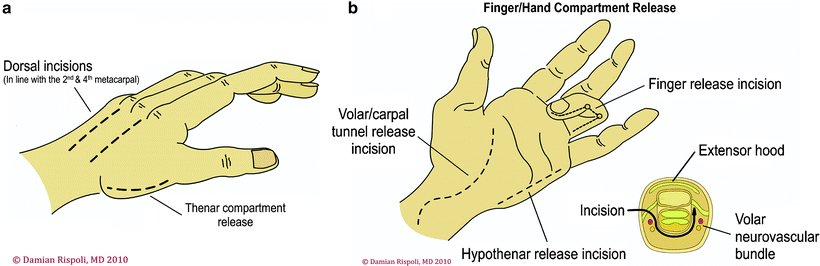
Fasciotomy of the Hand
The hand has a unique anatomy with ten separate fascial compartments: four dorsal and three volar interossei, thenar muscles, hypothenar muscles, and adductor pollicis. The fasciotomy technique consists of four incisions (Fig. 38.7). One incision on the radial side of the thumb metacarpal releases the thenar compartment. A dorsal incision over the index finger metacarpal is used to release the first and second dorsal interossei and to reach the ulnar-to-index finger metacarpal and to release the volar interossei and adductor pollicis. A dorsal incision over the ring finger metacarpal is used to release the third and fourth dorsal interossei and to reach down along the radial aspect of the ring finger and small finger metatarsal to release the volar interossei. An incision placed at the ulnar aspect of the small finger is used to release the hypothenar muscles.