Grade
PaO2/FiO2 (mmHg)
Radiographic infiltrates consistent with pulmonary edema
0
>300
Absent
1
>300
Present
2
200–300
Present
3
<200
Present
Lung Transplantation
The primary mode of ECLS following lung transplantation is VV ECMO. VV ECMO provides direct support of gas exchange, facilitates lung rest, and, indirectly supports cardiac function. While the direct consequence of PGD is impaired gas exchange and poor lung compliance, right ventricular (RV) dysfunction may be a significant contributor to the patient’s cardiorespiratory state. Many patients undergoing lung transplantation have preexisting RV dysfunction due to chronically elevated pulmonary vascular resistance (PVR) . Hypoxia, hypercarbia, and acidemia arising as a consequence of PGD lead to further increases in PVR during the postoperative period. Elevated intrathoracic pressure, secondary to high peak inflation pressure (PIP) and high positive end-expiratory pressure (PEEP), increase RV afterload and contribute to RV dysfunction. Finally, cardiopulmonary bypass (CPB) , if utilized for surgery, greatly increases the sensitivity of the pulmonary circulation to the effects of hypercarbia [16, 17]. While VV ECMO provides no direct support of cardiac function, improved gas exchange and application of rest ventilator settings typically leads to improved RV function. VA ECMO only rarely required following lung transplantation, but is occasionally necessary for patients with severe RV dysfunction.
Indication for ECMO Following Lung Transplantation
PGD may present immediately following graft reperfusion or develop more slowly over the first few hours. One or both transplanted lungs may be involved. In the operating room, PGD typically presents acutely with the appearance of pulmonary edema fluid in the endotracheal tube following reperfusion of the graft. PGD that is bilateral may result in an acute failure of gas exchange and the immediate need for ECLS. In the intensive care unit (ICU), PGD typically evolves more slowly over several hours with deteriorating gas exchange, worsening lung compliance, and alveolar shadowing on the chest radiograph.
Before diagnosing PGD it is essential to exclude pulmonary venous obstruction or obstruction within the airways. The pulmonary veins should be examined with transesophageal echocardiography (TEE) looking for signs of kinking or obstruction (Fig. 16.1). Pulmonary venous obstruction should be treated with immediate surgical revision. Obstruction within the airways can arise from mucus plugs or blood clots. Fiberoptic bronchoscopy should be performed, and any mucus plugs or blood clots removed under direct vision.
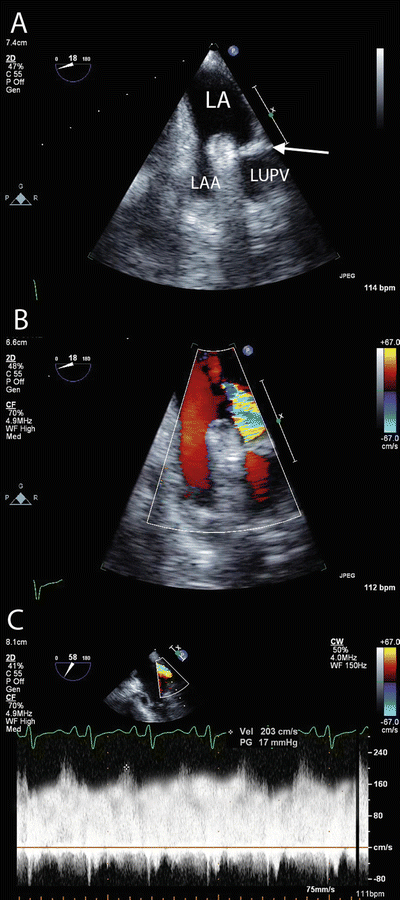

Fig. 16.1
Pulmonary venous obstruction . (a) TEE imaging of the left upper pulmonary vein and left atrial appendage demonstrating obstruction (arrow) within the pulmonary vein. (b) Color Doppler imaging demonstrating turbulent flow at the site of obstruction. Flow in the pulmonary veins is normally laminar (similar to the flow in the adjacent atrial appendage). (c) Pulsed Wave Doppler imaging demonstrating increased velocity (203 cm/s) and loss of the normal phasic flow pattern within the pulmonary vein. The normal peak velocity of blood flow in the pulmonary veins is <1 m/s. LA left atrium, LAA left atrial appendage, LUPV left upper pulmonary vein
Conventional treatment of PGD involves lung-protective ventilation with increased PEEP (5–15 cm H2O), inhaled pulmonary vasodilators (nitric oxide, prostacyclin), vasopressor and inotropic therapy to support RV function, fluid restriction, and correction of metabolic acidosis with renal replacement therapy. Lung protective ventilation involves limiting plateau airway pressure to 30 cm H2O or less (or PIP ≤ 35 cm H2O), using low tidal volume (VT) breaths (≤6 mL/kg), and accepting a degree of hypercarbia and respiratory acidemia. This approach improves survival in patients with acute respiratory distress syndrome (ARDS) [18]. While not of proven benefit, lung protective ventilation is also utilized following lung transplantation in the hope of minimizing PGD, and thereby potentially increasing survival and improving graft function over the longer-term. While permissive hypercarbia (PaCO2 50–70 mmHg, pH 7.1–7.2) is normally well tolerated in patients with ARDS, in lung transplant recipients this strategy may precipitate acute RV failure due to the adverse effect on PVR.
ECMO should be instituted if adequate gas exchange (PaO2/FiO2 > 80 mmHg, PaCO2 < 60–70 mmHg, pH > 7.2) cannot be maintained despite maximal lung protective ventilation (PIP 35 cm H2O, PEEP 15 cm H2O, respiratory rate [RR] 25/min, FiO2 > 0.6), or if the hemodynamic state is deteriorating (mean arterial pressure [MAP] < 60 mmHg, central venous pressure [CVP] > 15 mmHg, cardiac index [CI] < 2.0 L/min/m2).
Technical Aspects of VV ECMO Following Lung Transplantation
Circuits and Cannulation for VV ECMO
Several cannula configurations may be used for VV ECMO (Fig. 16.2). Our preferred configuration is femeroatrial (Fig. 16.2a). For drainage, a long 25–29 French (Fr) multiport cannula is placed in the femoral vein and advanced so the tip lies in the inferior vena cava (IVC) just below the hepatic vein, 5–10 cm from the junction of the IVC and right atrium (RA) (Fig. 16.3). For return, a short 19 Fr cannula is introduced into the right internal jugular vein and advanced so the tip lies just proximal to the junction of the RA and superior vena cava (SVC). This arrangement typically allows a circuit flow in excess of 6 L/min. A commonly used alternative to femeroatrial cannulation is to use a purpose-designed double-lumen ECMO cannula (Avalon Elite Bi-Caval Dual Lumen Catheter, MAQUET, Rastatt, Germany) inserted into the right internal jugular vein (Fig. 16.2c). The tip of the drainage lumen sits in the IVC at about the level of the hepatic vein, while the return lumen opens into the RA. For adults a 27 or 31 Fr cannula typically allows flows up to 5 L/min.
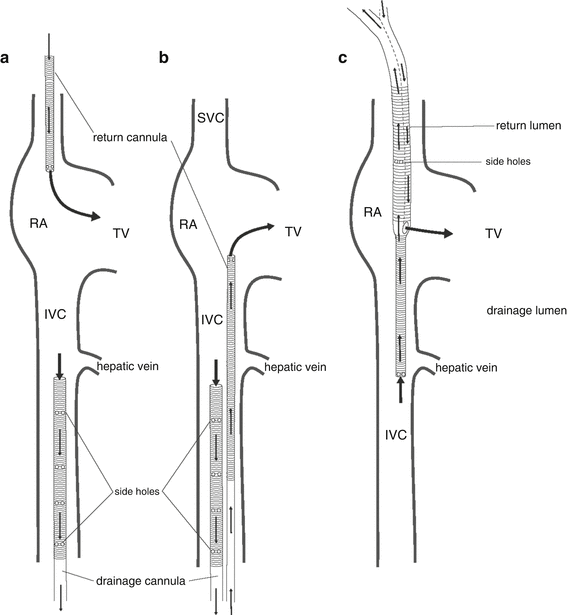
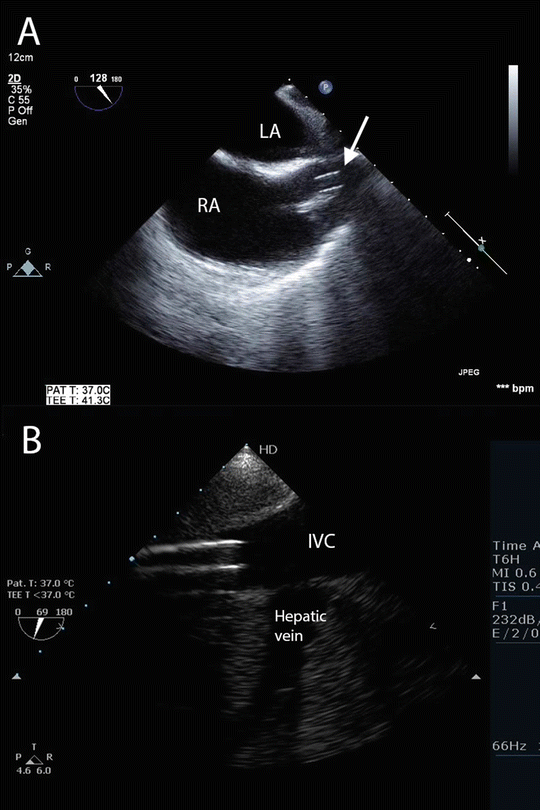

Fig. 16.2
Common cannula configurations for VV ECMO . (a) Femeroatrial cannulation. Drainage is via a large multiport cannula introduced into a femoral vein and advanced to the mid-inferior vena cava; return is via a short cannula introduced into the right internal jugular vein and advanced to the proximal superior vena cava. (b) Femerofemoral cannulation. Drainage is via a large multiport cannula introduced into a femoral vein and advanced to the mid-inferior vena cava; return is via a long cannula introduced into the contralateral femoral vein and advanced to the right atrium. (c) Double-lumen cannulation. Drainage and return are via a double-lumen cannula introduced into the right internal jugular vein. The cannula is advanced until the tip lies in the mid-inferior vena cava, just distal to the hepatic vein. Drainage is from the inferior and superior vena cavae. Return is to the right atrium. RA right atrium, TV tricuspid valve, IVC inferior vena cava. (From Sidebotham et al. [20]; with permission.)

Fig. 16.3
Normal cannulae position for VV ECMO using femeroatrial arrangement (see Fig. 16.1a). (a) TEE imaging from the mid-esophageal bicaval view showing the tip of the return cannula (arrow) in the superior vena cava. (b) TEE imaging of the inferior vena cava and hepatic vein. The tip of the drainage cannula is seen in the inferior vena cava just distal to the origin of the hepatic vein. Compare to the arrangement for VA ECMO shown in Fig. 16.6. LA left atrium, RA right atrium, IVC inferior vena cava
EMCO cannulae may be inserted by a surgical cutdown or a Seldinger technique. A particular advantage of the Seldinger technique is avoidance of cannula-site bleeding that often occurs with surgical cutdown. Peripheral cannulation through the jugular and femoral veins is preferred to central cannulation through the wound, as this approach allows closure of the chest wound, minimizing surgical-site bleeding and reducing the risk of mediastinal infection. The position of guide wires and cannulae should be confirmed with TEE. Once ECMO has commenced, flow in the return cannula should be interrogated with color Doppler imaging to ensure it is directed through the tricuspid valve (see below) (Fig. 16.4).
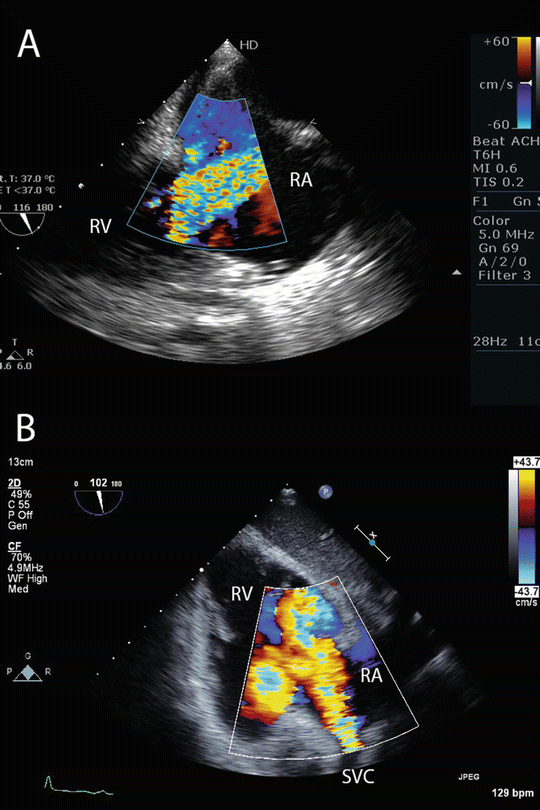

Fig. 16.4
Normal flow pattern of the return cannula during VV ECMO . (a) TEE imaging from a modified mid-esophageal bicaval view showing a high velocity jet passing from the right atrium, through the tricuspid valve, into the right ventricle. (b) TEE imaging from a transgastric view of the right ventricle. A high velocity jet can be seen originating from the superior vena cava, and passing into the right atrium and ventricle. In both images, the jet of oxygenated blood passes through the tricuspid valve. RA right atrium, RV right ventricle, SVC superior vena cava
Modern ECMO circuits are comprised of a polymethylpentene (PMP) oxygenator, a centrifugal pump, heparin coated tubing, a pump controller, a heater unit, a gas blender, and sampling ports (Fig. 16.5). Compared to older-style circuits comprising roller pumps and hollow fiber or silicone oxygenators, modern circuits are more durable, cause less damage to blood components, and provide more efficient gas exchange [19, 20].
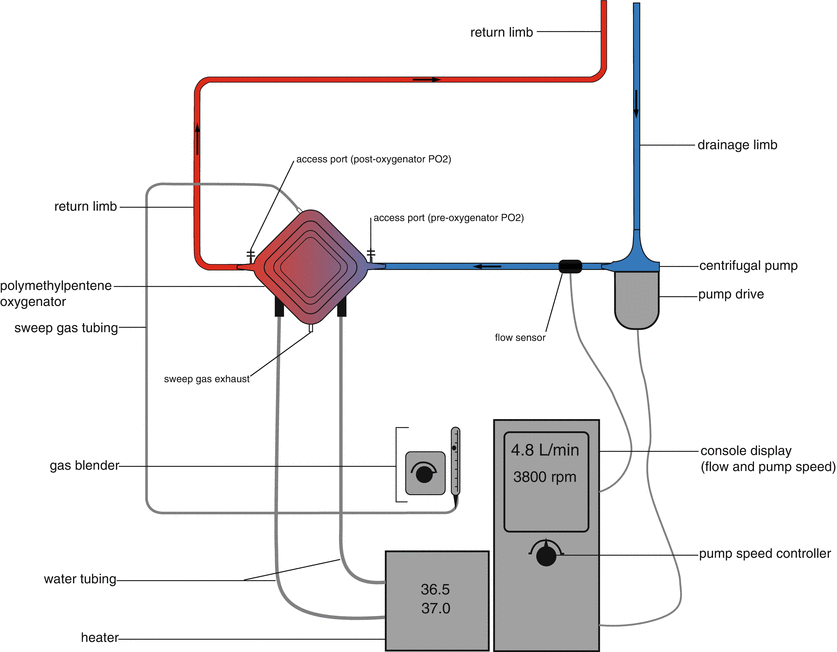

Fig. 16.5
Schematic of an ECMO circuit comprised of a polymethylpentene oxygenator, centrifugal pump, pump drive, pump controller, water heater, and gas blender. (Image modified from Sidebotham et al. [20]; with permission.)
Gas Exchange During VV ECMO
During VV ECMO, deoxygenated blood is drained from the IVC and oxygenated blood is returned to the RA. Ideally, all the blood from the return cannula passes through the tricuspid valve into the pulmonary circulation. Because oxygenated blood from the return cannula mixes with deoxygenated blood from the patient’s systemic venous return, it is not possible to achieve a normal SaO2 with VV ECMO. However, if ECMO circuit flow is at least 70 % of cardiac output, and the majority of the ECMO return blood passes into the pulmonary circulation, a SaO2 in the range 88–92 % can be achieved even if the lungs are not contributing to gas exchange [20].
The main determinant of SaO2 during VV ECMO is circuit flow. Low SaO2 (<88 %) despite adequate circuit flow (4–6 L/min.) may be caused by recirculation, abnormally high cardiac output (e.g., due to sepsis), or oxygenator failure. Recirculation occurs when oxygenated blood from the return cannula passes directly to the drainage cannula. Measuring the pre-oxygenator SO2 in the circuit helps distinguish between recirculation and high cardiac output as the cause of hypoxemia. A high pre-oxygenator SO2 (>75 %) suggests recirculation, whereas a low pre-oxygenator SO2 (<60 %) suggests high cardiac output.
The severity of recirculation is influenced by intravascular volume, ECMO flow, and, most importantly, by the relative positions of the drainage and return cannulae. If recirculation is suspected, a TEE examination should be performed to assess the position and flow patterns of the ECMO cannula (Fig. 16.6). If recirculation is confirmed the cannulae positions should be adjusted under TEE guidance.
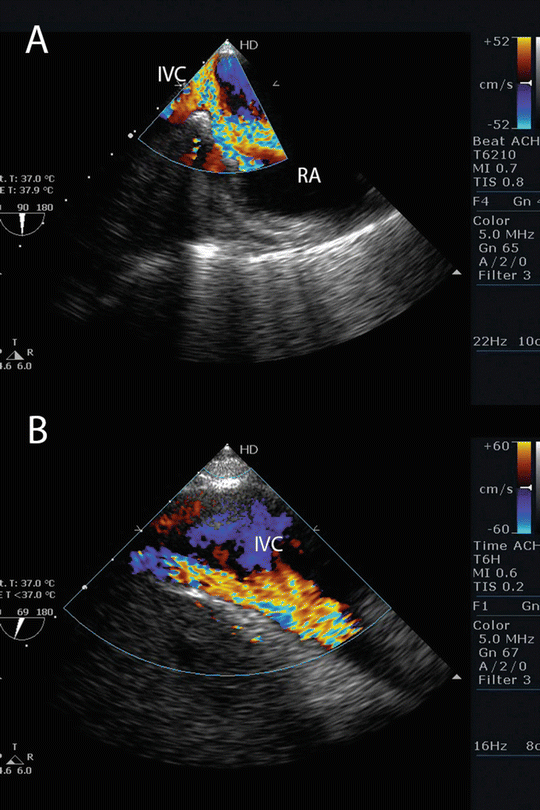

Fig. 16.6
Recirculation during VV ECMO . (a) TEE imaging from a modified bicaval view showing the jet of blood from the return cannula passing from the right atrium into the inferior vena cava. (b) TEE imaging in the same patient demonstrating flow in the inferior vena cava from the return cannula. In this patient, oxygenated blood in from the return cannula is likely to be entrained into the drainage cannula (not seen) leading to recirculation. RA right atrium, IVC inferior vena cava
PaCO2 is not influenced by circuit flow but by the balance between the sweep gas flow and the patient’s metabolic state (i.e., CO2 production). Normocarbia can usually be achieved with a sweep gas flow of 1–2 times the circuit blood flow.
Common problems and their potential solutions during ECMO support are listed in Table 16.2.
Table 16.2
Problems and solutions during extracorporeal membrane oxygenation
Problem | Possible causes | Diagnosis | Solution |
|---|---|---|---|
Arterial hypoxemia (VV) | Low circuit flow Recirculation High cardiac output/increased metabolic rate Oxygenator failure | Check pre-oxygenator SO2 Check post-oxygenator SO2 TEE examination | Increase circuit flow Adjust cannulae position Replace oxygenator Active cooling |
Arterial hypoxemia (VA) | Excessive ejection from LV in patient with pulmonary dysfunction Oxygenator failure | Compare SPO2 in arm/leg Check post-oxygenator SO2 | See text for treatment of upper-body hypoxemia Replace oxygenator |
Hypercarbia (VV, VA) | Inadequate sweep gas flow Increased metabolic rate Oxygenator failure | Check post-oxygenator PaCO2 | Increase sweep gas Cool patient Replace oxygenator |
Hypotension (VV) | Cardiac failure Vasoplegia | TEE examination | Inotrope or vasopressor Convert to VA ECMO |
Hypotension (VA) | Vasoplegia Tamponade | TEE examination | Vasopressor Surgical exploration |
Suction events (VA, VV) | Hypovolemia Malpositioned cannulae Tamponade (VA) | TEE examination | Give fluid Adjust cannulae Surgical exploration |
Low pump flow /high pump speed (VA, VV) | Hypovolemia Obstruction in circuit (oxygenator/pump) Malpositioned cannulae Tamponade | Examine circuit for clots Check pressure drop across oxygenator TEE examination | Give fluid Replace circuit component Surgical exploration |
Ventricular distension or intracardiac thrombus (VA) | Inadequate LV ejection Inadequate heparinization | Clinical signs of pulmonary edema TEE examination | Increase inotropes Increase circuit flow Create ASD |
Heparin resistance (VA, VV) | Anti-thrombin III deficiency | High heparin dose requirements (>20 U/kg/h) | Give fresh plasma or recombinant antithrombin III |
High plasma hemoglobin (VA, VV) | Thrombus in pump or oxygenator | Examine circuit Measure pressure drop across oxygenator | Replace circuit component |
Adjuvant Therapy During ECMO Support [19–21]
Once EMCO has commenced the ventilator should be set to rest settings to minimize further ventilator-induced lung injury. Typical rest settings are a FiO2 0.4, PIP 20 cm H2O, PEEP 10 cm H2O, and a RR of 10/min.
Systemic anticoagulation with unfractionated heparin is routine during ECMO to prevent thrombus forming in the circuit. However, in the early postoperative period, when surgical-site bleeding is a concern, is may be appropriate to run the circuit heparin-free. ECMO can usually be managed heparin-free for several days without adverse consequences. Once postoperative bleeding has settled the patient should be fully heparinized. Traditionally heparin anticoagulation during ECMO has been monitored by measuring the activated partial thromboplastin time (APTT) . However, because APTT readings are highly variable between different laboratories and because APTT levels are influenced by factors other than heparin anticoagulation, many centers have moved to titrating heparin dose to the antifactor Xa level, aiming for a value of 0.3–0.7 U/mL [22]. Marked heparin resistance (heparin dose requirement >20 IU/kg/h) is suggestive of low antithrombin III (ATIII) levels. ATIII levels below 50 % of normal in the presence of high heparin requirements should be treated with fresh frozen plasma or recombinant ATIII. Bleeding is an ever-present risk while patients are anticoagulated for ECMO. If possible, invasive procedures (e.g., tracheostomy, intercostal drainage of simple pneumothoraces) should be deferred until ECMO has been discontinued.
To promote graft recovery a restrictive approach to fluid therapy and regular diuretic therapy is appropriate. Early institution of renal replacement therapy for control of metabolic acidosis and acute kidney injury is indicated. Strict attention to asepsis is essential as the combination of immunosupression, critical illness, and invasive cannulae all increase the risk of nosocomial infection. Two strategies of proven benefit that are particularly applicable to ECMO-supported patients are daily bathing with chlorhexidine-impregnated washcloths [23] and the use of chlorhexidine dressings for covering vascular (including ECMO) catheters [24]. Careful attention to hand hygiene, early institution of enteral nutrition, and daily circuit cultures are also important.
Weaning and Discontinuing ECMO
Signs of pulmonary recovery are indicted by reduced ECMO flow to maintain SaO2, an improving chest radiograph, and increased tidal ventilation. When the required circuit flows to maintain a SaO2 above 92 % has reduced to less than 3–4 L/min and tidal volume on rest ventilator settings have increased to more than 200 mL, a trial on standard ventilation (FiO2 0.4, PIP 25–30 cm H2O, PEEP 5–10, RR 15 breaths/min.) off extracorporeal support is indicated. Leaving the circuit flow at 3–4 L/min but turning the sweep gas off keeps the circuit patent but provides a trial off ECMO support. If, after 2–4 h, tidal volume and gas exchange are adequate (i.e., VT > 4–5 mL/kg, PaO2 > 80 mmHg, PaCO2 < 60 mmHg) ECMO may be discontinued and the patient decannulated. If an unmodified Seldinger technique has been used for insertion, the cannulae can be removed and manual pressure applied to the insertion site for 10–15 min. If the cannulae were inserted by a cut-down technique, surgical removal and repair of the veins is indicated.
Outcome from ECMO Following Lung Transplantation
Approximately 3–6 % of patients receive ECMO following lung transplantation [25–29]. Early survival is 50–80 % [25, 26, 30], which compares to more than 90 % for lung transplant recipients overall [9]. Longer term outcome is also worse in patients requiring ECMO. In one study, 3-year graft survival was 49 % in ECMO-supported compared to 74 % in non-ECMO-supported patients [25]. Follow up peak forced expiratory volume in 1 s was 58 % of predicted in the ECMO group compared to 88 % in the non-ECMO group.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






