Chapter 53 Extracorporeal Life Support
Aided by the discovery of heparin in 19161 and by advances in the technology of membrane oxygenators, extracorporeal life support has changed dramatically since the early 1950s, when John Gibbon first used a machine of his own design to provide extracorporeal life support for a cat whose pulmonary artery was occluded with a clamp.2 Early experiences with the use of cardiopulmonary bypass for operations on the heart were mixed, but as experience and technology have continued to advance, the field of cardiopulmonary bypass has expanded at a rapid rate.3–5 Today the dreams of clinicians like Gibbon have been realized, and extracorporeal support for the heart and lungs is used daily throughout the world. Nowhere is the impact of cardiopulmonary bypass seen more clearly than in pediatrics, where increasingly intricate intracardiac repairs of once-lethal congenital heart defects are performed every day with the aid of cardiopulmonary bypass.
As experience with bypass techniques in the operating suite grew, investigation of the use of extracorporeal support of patients with cardiopulmonary failure outside the operating room began.6,7 Infants with severe respiratory disease or pulmonary hypertension were among the groups for whom use of a temporary cardiopulmonary bypass system seemed appropriate. This technique of modified cardiopulmonary bypass came to be known as “extracorporeal membrane oxygenation” (ECMO).8 Although premature infants had an unacceptably high incidence of intracranial hemorrhage as a result of the systemic heparinization, infants of more than 35 weeks’ gestation with respiratory failure were successfully supported with ECMO.9,10 An abandoned infant with severe hypoxemia named “Esperanza” (hope) by her caregivers was among the first to be treated with ECMO by Bartlett in 1976.10a Today, Esperanza is a grown woman with children of her own. Efforts to organize and collate data on patients treated with ECMO resulted in the formation of the Extracorporeal Life Support Organization (ELSO). In 2009, ELSO celebrated its 20th anniversary. This largely volunteer network of physicians, surgeons, nurses, respiratory therapists, and all those with an interest in extracorporeal life support comprises more than 100 centers and contains data on more than 40,000 patients treated with extracorporeal life support throughout the world.11
As more patients have been treated and techniques have been refined, ECMO procedures and management of patients have evolved to the point that ECMO now can be offered to patient groups previously excluded from consideration.12–14 Despite multiple attempts to define specific selection criteria for ECMO candidates, no well-defined and universally applied criteria exist. The decision regarding when a patient should be treated with ECMO remains empirical and based on experience. Similarly, although there is little complete standardization of ECMO circuit design, cannulation technique, and patient management, the general principles are fairly constant. Guidelines for ECMO center training, equipment selection, and patient selection and management recently have been developed by expert consensus and are posted on the ELSO Web site. Although the guidelines are fairly general, it is hoped that they will enable more standardization of practice in the future. The information in this chapter represents general practice, the authors’ experience, and a review of the literature. For more detailed information regarding extracorporeal life support, the reader is directed to the excellent text regarding this subject published by the ELSO organization.10a
Materials and Methods
Cannulation Techniques
Venoarterial Extracorporeal Membrane Oxygenation
Venous Access
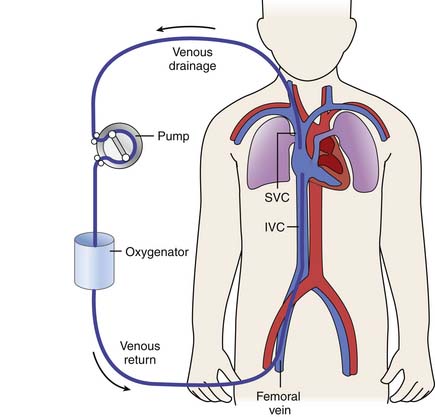
In venoarterial ECMO, desaturated systemic venous blood is drained from the body and reinfused into a large artery after being oxygenated in the ECMO circuit (Figure 53-1). The right atrium is the usual site accessed for ECMO cannulation. The internal jugular (IJ) vein is a large vessel with a fairly short, straight course to the right atrium and thus is preferred during cervical cannulation. To augment cerebral venous drainage in patients who undergo cannulation via the right IJ vein, some centers also place a smaller cannula retrograde in the vessel to the level of the jugular venous bulb at the base of the skull. This catheter can facilitate cerebral venous drainage and provide a means of monitoring jugular venous oxygen saturation. Some clinicians believe that monitoring jugular venous saturation provides information on adequacy of oxygen delivery to the brain and that it is valuable during ECMO, although this practice is relatively controversial. Reports of an increase in venous drainage via the retrograde cannula of up to one third have been noted in some centers.15 The retrograde catheter is connected by a Y-adapter into the larger venous drainage line.16,17
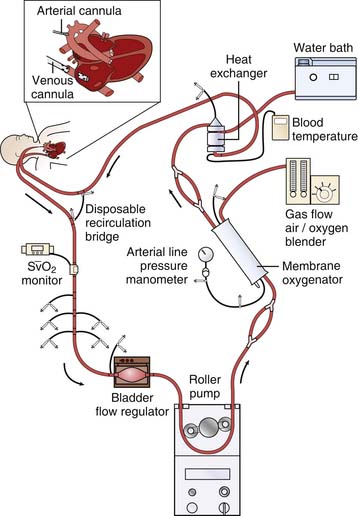
Figure 53–1 Venoarterial extracorporeal life support via cervical cannulation. SvO2, Venous oxygen saturation.
(M. Dowhy, with permission. Courtesy Children’s Hospital, Pittsburgh, Pa.)
In older children and adults, the femoral vessels can be used for cannulation.18 Venous access is obtained from the saphenous or femoral vein into the inferior vena cava (IVC), or the cannula can be advanced further up the IVC to the right atrial/IVC junction. Although femoral vein cannulation diverts less venous return to the pump than a catheter positioned in the right atrium, the amount of blood drained is often adequate to meet the needs of the patient. Femoral cannulation is generally restricted by age and size to adolescents or adults. Some surgeons suggest that if the child is old enough to walk and run, the femoral vessels are of adequate size to consider femoral cannulation. One author (H.J.D.) has successfully used femoral vessels in a 13-kg 6-year-old child without difficulty. For patients with femoral venous access who exhibit venous stasis or obstruction of the extremity distal to the cannula, a similar catheter to that used for jugular venous drainage can be placed down the leg via the saphenous or femoral vein to augment decompression of the leg and augment venous return. This catheter is then connected by a Y adapter into the venous drainage line of the ECLS circuit. Compartment syndrome from venous stasis in femoral venous cannulation has been described, and thus careful monitoring of the extremity is mandatory.19
In patients who undergo cannulation via the mediastinum, the venous cannula is often placed directly into the right atrial appendage.20 Although other vessels, such as the subclavian vein or left IJ vein, have been used for ECLS, they have been associated with limb perfusion abnormalities or difficulties with adequate blood flow.
Arterial Access
The femoral artery also can be used for access during venoarterial ECMO. If a long femoral artery cannula that reaches the thorax is used, good oxygenation to the upper body is assured, but resistance to flow will be elevated.21 If a short femoral artery cannula is used, it normally sits in the iliac vessels. The amount of arterial return reaching the upper body, heart, and brain in this mode of ECMO is dependent on antegrade flow out of the native left heart and the retrograde flow from the ECMO arterial return. In patients with severe cardiac dysfunction, arterial return from the ECMO circuit flows further up the aorta and may predominate. In patients with good cardiac function, the majority of upper body arterial flow may be from native left heart ejection. Arterial flow from low-lying ECMO cannulas with good native heart function may thus preferentially flow to the lower body. This arterial flow will mix with venous return from the lower body before the oxygenated blood flows through the cardiopulmonary circuit and out the aorta. In patients with impaired gas exchange, the amount of venous mixing prior to reaching the ascending aorta reduces the amount of oxygen that is delivered to the upper body (heart and brain). Thus the PaO2 and arterial oxygen saturations in the upper body may be lower than that obtained with cervical venoarterial ECMO. Concern over limited oxygen delivery to the brain has resulted in slow acceptance of venoarterial ECMO with a low-lying femoral arterial cannula. Successful use of ECMO has been achieved, however, with this mode. Monitoring obtained oxygenation with a pulse oximeter on the right hand, ear, or nose of the patient is a good safety precaution to assess the amount of oxygenated flow getting to the head during this mode of ECMO. Echocardiography often can determine the extent of retrograde aortic flow versus native heart ejection. Femoral arterial cannulation also can be associated with impaired flow to the distal limb and resultant ischemia. A small “feeder” cannula can be directed distally down the leg artery and then connected by a Y adapter into the arterial cannula to improve perfusion. Additionally, placement of a 14-gauge catheter into the posterior tibial artery that is then connected by a Y adapter into the arterial side of the ECMO circuit also can provide distal limb perfusion. Ischemia and the need for amputation with femoral artery cannulation has been reported, and thus meticulous attention to limb perfusion is required to prevent this complication.
In mediastinal cannulation, the arterial return cannula is usually placed into the aortic arch under direct vision.22 During mediastinal cannulation, patients with severe left ventricular dysfunction who cannot open the aortic valve to eject blood often have a left atrial venting catheter inserted to allow decompression of the left heart. This technique prevents pulmonary venous hypertension, which can lead to severe pulmonary edema or hemorrhage. This catheter can be connected by a Y adapter into the venous drainage of the ECLS circuit to provide adequate left heart decompression. Patients with intact sternums who require left atrial decompression often are taken to the cardiac catheterization suite for a blade atrial septostomy that then allows the left heart to decompress into the right atrium and the blood to be drained into the venous ECMO cannula.23
Other arterial access sites such as the subclavian also have been used but have resulted in difficulty with limb perfusion. One recent report described the successful use of the axillary artery by means of a Gore-Tex graft placed into the side of the vessel for venoarterial support in adult lung transplant patients supported by ECMO.24
The primary modality to verify proper cannula position has traditionally been chest radiography. In a recent report comparing echocardiography and chest radiographs for evaluation of cannula placement during pediatric ECMO, 12% of patients had an abnormal cannula position noted with ECHO that was not identified on a chest radiograph. An additional 7% of patients were noted to have an abnormal cannula position during incidental echocardiography for other reasons. Any concerns with cannula position should be clarified by an echocardiographic examination.25
Venovenous Extracorporeal Membrane Oxygenation
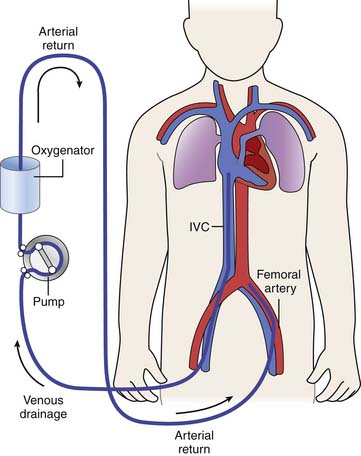
Venovenous ECMO differs from venoarterial ECMO in that blood is both withdrawn and reinfused into the patient’s venous circulation (Figure 53-2). Cannulation can be introduced via either the cervical or femoral vessels.26 Currently several types of multiple lumen, single cannulas exist. One such cannula, manufactured by Origen Inc (Austin, Tex.), is available in sizes from 12 to 18 Fr and can support patients weighing up to 12 kg. This cannula is placed into the right IJ vein and requires only one surgical site. The drainage and infusion lumens in this cannula are separated by a distance of a few centimeters. Careful placement and orientation of the cannula can reduce recirculation of reinfused blood from the ECMO circuit, although some amount of recirculation (which will be discussed later in further detail) always occurs. A new venovenous cannula manufactured by Avalon Inc also has become available in sizes from 13 to 31 Fr and is able to obtain flow rates to support even large adult patients. This cannula has two drainage lumens, one of which is positioned in the IVC and one in the superior vena cava. A reinfusion port that sits between the two lumens is directed at the tricuspid valve. Placement of this cannula requires meticulous assessment with echocardiography or fluoroscopy for optimal performance. Although experience with the Avalon cannula is not very extensive at this time, it has the advantage of reducing the need for multiple venous cannulation sites. Time will tell if the advantages of this cannula outweigh its increased cost. Preliminary results are encouraging.
Venovenous ECMO also can be provided via two (or more) separate access sites. The right IJ and femoral vein provide access in the majority of patients. Patients with venovenous cannulation may have venous blood drained from the right atrium via the IJ vein or from the IVC via the femoral vein. Although more venous drainage usually can be obtained from a cervical cannula (which is usually shorter and larger than a cannula that can be placed into the femoral vein), the femoral site often may prove adequate. Older children and adults also may undergo bilateral saphenous or femoral cannulations, with one cannula placed into the high IVC and the other ending in the low IVC or iliac vessels27 (Figure 53-3).
Several features unique to venovenous cannulation are important to understand. First, because blood is both withdrawn and reinfused into the venous circulation, adequate native cardiac function must exist to provide the “pumping” of oxygenated ECMO return to the patient’s systemic circulation. One factor that may influence cardiac function during venovenous ECMO is that well-oxygenated blood returning from the ECMO circuit will enter the right heart. This highly oxygenated blood may reduce pulmonary artery pressure by reducing pulmonary vascular resistance, which may in turn improve right heart function.28 Likewise, highly saturated blood ejected from the left ventricle to the coronary arteries may improve myocardial blood flow and improve cardiac performance. For these reasons, some clinicians will initiate venovenous ECMO even in patients with cardiac dysfunction and transition to venoarterial ECMO if support is inadequate. Other clinicians prefer to use venoarterial ECMO preferentially if cardiac dysfunction exists.
Another feature of venovenous ECMO is that because blood is withdrawn and reinfused into the venous side of the circulation, some of the return coming from the ECMO circuit will be drained by the venous drainage cannula before it goes through the patient’s heart into the systemic circulation. This phenomenon is known as “recirculation,” and it can be a major limiting factor in providing adequate patient support with venovenous ECMO.29 With double-lumen cannulas placed via the right IJ vein to the right atrium, careful orientation of the inflow lumen toward the tricuspid valve may limit recirculation. The larger separation of drainage and inflow lumens with the Avalon device seems to be associated with fewer recirculation difficulties.
With two-site venous cannulation, recirculation can be limited to some degree by ensuring that some distance separates the end tips of the drainage and infusion cannulas in the body. Recirculation also can be reduced by draining from the femoral vein cannula and reinfusing into the right IJ vein cannula. The extent of recirculation can be estimated by following the venous saturation in the ECLS circuit; high levels of recirculation will elevate the displayed venous saturation on the drainage line because some of the highly saturated return from the ECLS circuit is immediately drawn out by the drainage cannula. Whether venovenous cannulation will provide adequate capture of the patient’s cardiac output for ECMO support depends on how large the cannulas are, where they lie in the vessel, and how much overall ECMO support the patient requires.30
Percutaneous Cannulation
Although the historical approach to vessel access has been via an open procedure with placement of cannulas under direct vision, kits for percutaneous placement now exist in many sizes. These kits use a modified Seldinger technique with obturators increasing in size to dilate the vessel. Once the appropriate size is reached, the cannula is passed into the vessel over the largest obturator.31
Percutaneous cannulation carries with it the inherent risks of potentially tearing a large vessel during cannulation. Although percutaneous cannulation in some centers is performed by nonsurgical personnel, surgical backup to perform an immediate cutdown for control of bleeding from a disrupted vessel may be needed. Despite the obvious fear of vessel disruption, this complication occurs infrequently. In one early series of 21 patients who underwent cannulation percutaneously, only 18% required any purse-string suturing around the cannulation site for bleeding, and none experienced vessel disruption.32 Percutaneous cannulation avoids the need for an open surgical site, thus decreasing surgical site bleeding and risk of infection.
Decannulation
Decannulation involves removal of surgically placed cannulas and repair of the operative site, with or without vessel repair. Vessels used in traditional, open venotomy or arteriotomy ECMO often can be repaired at decannulation, although this approach is not used universally. Whether avoiding ligation or repairing cannulated vessels results in long-term improvement in blood flow or reduced risk of stroke from thrombosis or infarct is unknown.33–37 The longest follow-up study of repaired carotid vessels found restenosis or occlusion in 24%. Patients with reconstructed vessels had fewer neuroradiologic abnormalities and a smaller incidence of cerebral palsy than did unrepaired historical control subjects, although these data represent a small, single-center report and are not the result of a randomized study.38 Femoral vessels are sometimes accessed via a Gore-Tex or similar graft sewn into the side of the vessel. This graft is tied off at decannulation.
Venous Reservoir and Venous Saturation Monitor
Following cannulation, venous ECMO blood flows by gravity through the attached tubing to a reservoir called the “bladder.” The bladder historically is a 30- to 50-mL oblong device that sits at the lowest point of the ECMO circuit and allows blood to collect and be drawn from it to the pump-head. It also acts as an air trap and normally has access ports to allow aspiration of any air collected in the bladder chamber. The output of the ECMO circuit is determined primarily by the amount of venous blood that can be withdrawn from the patient to enter the circuit. The venous drainage to the bladder is gravity dependent, generated by the pressure difference between the column of blood in the venous cannula and the reservoir. Thus the siphon effect of venous return is augmented by elevating the patient higher above the venous reservoir.39 Because the venous cannula can drain only as much blood as is available at the cannulation site, adequate systemic venous return must be maintained. In addition to systemic hypovolemia, any process that limits right atrial filling, such as pneumothorax or pneumopericardium, will also impede drainage to the ECMO circuit.40 Changes in venous and arterial tone also may alter right atrial and venous filling pressures and affect the amount of venous drainage that can be obtained. The bladder can be used as a servoregulating device that helps to match forward flow to venous return. Advancement in bladder technology has resulted in the Better Bladder (Circulatory Technology Inc, Oyster Bay, NY), which is a piece of collapsible tubing encased in a hard shell. Changes in venous return cause contraction of the tubing, which is registered as a change in negative venous pressure in the circuit. This device provides servoregulation of flow. The vertical orientation of the bladder reduces clotting compared with older oblong, horizontal devices. Servoregulation by monitoring of direct negative and positive pressure within the ECMO circuit has eliminated the use of the bladder in some centers, although most still incorporate some sort of “bladder” within the circuit.
Types of Pumps and Oxygenators
Roller-Head Pumps
The majority of ECMO centers use a roller-head pump during extracorporeal support. This device contains steel roller heads enclosed in a box (the pump housing) (Figure 53-4). The heads rotate and push against the ECMO circuit tubing, which is threaded through the box. The piece of tubing in contact with the roller heads is called the raceway and is generally composed of a special material called super-Tygon, which is less likely to rupture under the wear and tear induced by the roller heads. Blood contained in the tubing is advanced forward by the motion of the roller heads. The amount of blood advanced from the raceway is dependent on the number of revolutions of the roller heads, the tubing size, and the occlusion between the heads and the tubing. Too little contact between the roller heads and the tubing (loose occlusion) will decrease the amount of blood advanced, while excessive pressure of the roller heads against the tubing (tight occlusion) will obstruct blood from moving forward and cause excessive wear on the tubing, which may result in rupture. The correct occlusion is set by measuring the displacement of fluid during roller-head rotation during setup of the ECMO circuit. Newer pumps used for ECMO now often incorporate flow probes that display the actual amount of blood flowing through the ECMO circuit. Transonic probes also can be attached to the outside surface of the ECMO tubing to monitor flow.
Loss of venous return with continuous rotation of the roller heads results in generation of high negative pressure in the ECMO tubing that can lead to hemolysis and cavitation as air is drawn out of solution.41 The collapse of the tubing and cannula that can be induced by high negative pressure also can lead to damage of the endothelium of the vessel or the right atrium. To protect the patient as venous return is lost and the bladder starts to collapse, a signal is sent to the pump head that causes it to slow down or stop until adequate venous return is achieved and the bladder is once again full. Older versions of the servoregulation system stop the pump whenever venous return diminishes and then restart it when venous return is adequate. This on/off action of servoregulation has been shown to result in acute changes in cerebral blood flow in patients who have undergone cannulation via the cervical route, which may be harmful.42 Newer circuit and pump designs have eliminated the traditional “bladder box” in favor of the Better Bladder previously described, which performs a similar servoregulating function. The elimination of the bladder reduces the potential for clot formation. It also removes a potential site for air trapping, however, which was easily accomplished with the older cylindrical bladder design. In the new systems, as venous return to the ECMO circuit diminishes, the collapsible tubing signals the roller heads to slow down and then speed up again once adequate venous return is obtained. This mechanism may decrease the on/off action of the roller-head pump and potentially limit the acute changes in blood flow noted with the older style of servoregulation. Newer pumps also may be able to provide pulsatile flow, which may be useful in maintaining normal perfusion patterns to the body. The nonpulsatile nature of venoarterial ECMO flow has been implicated in the renal dysfunction sometimes noted in patients treated with venoarterial ECMO. The pulsatile flow of the newer pumps has yet to be effectively linked to native heart ejection, although this link is a goal in the future.
Centrifugal Pumps
Centrifugal pumps also are used for ECMO support (Figure 53-5). Advanced technology in this area has resulted in many centers changing from roller-head to centrifugal pumps. Some centrifugal pump heads contain a spinning rotor that is controlled by magnets within and without the cone.43,44 Blood enters the cone at the apex and is propelled tangentially to the base of the pump head, where it is expelled. The longer blood sits in the centrifugal head and the faster the head spins, the more hemolysis will be created. It is also postulated that as blood clots develop in the membrane oxygenator over time and increase outflow resistance, this scenario may also increase hemolysis within the rotor head. Hemolysis has limited the use of centrifugal pumps. Newer pump designs have small heads that require little priming volume and technical advancements in generating the “spinning” motion, which may be associated with less hemolysis than in the past. Use of low-resistance hollow fiber oxygenators also may reduce hemolysis and make centrifugal pumps even more efficient.
Blood flow to the centrifugal head is augmented by the “suction” effect of the spinning pump head and thus is not as dependent on gravity drainage as are roller-head pumps. Thus centrifugal pumps can be placed at any level relative to the patient, which makes transporting the patient who is being treated with ECMO easier than with roller-head devices. The active suction effect of centrifugal heads can create levels of negative pressures as high as −200 to −700 within the venous inlet tubing. High amounts of negative pressure generated by centrifugal pumps can result in hemolysis, endothelial damage of cannulated vessels, or cavitation of air. Monitoring of venous pressure with alarm limits to signal when excessive negative pressure is occurring is common in most centers. Monitoring venous return and understanding the function of centrifugal pumps are keys to safe and appropriate use of this form of support.45,46 Another advantage of centrifugal pumping devices is that air and debris are trapped within the vortex of the pump head and may be less likely to embolize to the patient than with roller-head circuits. This advantage is countered by some reports that centrifugal pumps generate more microbubbles than do roller-head devices. A recent publication examining causes of hemolysis noted that the direct interaction of blood with air and high negative pressure (such as occurs with cardiotomy suction during bypass surgery) resulted in the greatest amount of hemolysis.47 Centrifugal circuits contain a flow probe that displays how much blood is being returned to the patient. Servoregulation occurs by the same method as with roller pumps, in that venous and arterial pressure limits and goals are set and the pump (or the specialist) adjusts flow to maintain set goals. Another advantage of centrifugal pumps is that if there is occlusion of the circuit past the centrifugal head, no high back pressure is exerted to result in rupture of the circuit. Obstruction to forward flow, however, will result in increased hemolysis from the blood trapped in the centrifugal head. The components of ECMO circuits used with centrifugal pumps can be completely coated with heparin, which may limit bleeding or the need for exogenous heparin. Although centrifugal pumps are easy to set up and operate, lack of familiarity with them compared with roller-head pumps requires that close attention to patient physiology and pump interaction be maintained. The newer designs of centrifugal systems allow miniaturization such that transport systems can be more compact and much less cumbersome than in years past.
Advances in ACLS will likely be drawn from continuing work with artificial heart or ventricular support devices. One intriguing report from Japan noted successful use of a self-regulating ECMO device that contained two sac-type blood pumps that are placed in parallel and use compressed air to eject blood. This pump is completely self-regulated and provides pulsatile ECMO return. Three neonatal patients with respiratory failure were successfully supported in Japan with use of this device.48
Membrane Oxygenator
Gas exchange in the membrane oxygenator is determined by the permeability of the membrane to oxygen and carbon dioxide (their diffusion coefficients), the available membrane surface area, the pressure gradient for oxygen or carbon dioxide between the gas compartment and the blood, and the amount of time gas and blood interface across the membrane.49 Viscosity, temperature, and pH of the blood also can influence the amount of oxygen binding to the blood as it passes through the oxygenator. Each membrane has a predetermined maximum blood flow above which the thickness of the blood film will limit oxygenation. Blood or gas flow in excess of the manufacturer’s specifications may generate high pressure within the membrane oxygenator, which may cause it to rupture. If the blood side of the envelope ruptures, blood will escape into the gas side of the envelope and will drip out through the gas exit port at the bottom of the oxygenator (colloquially called a nosebleed). Small breaks in the integrity of the blood/gas barrier with little bleeding can be tolerated because the small tear will usually clot off. More vigorous bleeding often requires an emergent oxygenator change. Rupture of the gas side of the envelope from excessive gas pressure can result in air being transferred to the blood phase of the membrane. This scenario is a true emergency that requires immediate stopping of ECMO and replacement of the oxygenator. Although it is an uncommon complication, a massive air embolus and death can occur in this scenario.
Countercurrent gas and blood flows in the oxygenator provide optimal gas exchange by maintaining the pressure gradient for oxygen transfer from gas to blood along the entire length of the membrane surface. The gravitational gas flow through the membrane also helps remove the condensation that forms when warm blood flows by cooler gas in the membrane. Water droplets are expelled from the membrane surface via the gas exhaust port. Membrane failure can be characterized by a decrease in the capacity to remove carbon dioxide or to oxygenate blood. Membrane failure may result from clot formation on the membrane, which decreases the available surface area for gas exchange; excess condensation in the membrane lung (“pulmonary edema”); or technical defects in the membrane itself. A difference of greater than 100 torr between the estimated PAO2 in membrane gas and that in blood leaving the oxygenator may indicate early membrane failure. Membrane failure also can be predicted when the pressure drop between blood entering the membrane and leaving it increases and becomes >100 torr. This situation may indicate clotting in the membrane oxygenator with restriction to blood flow. Initially, increasing the partial pressure of oxygen (PO2) in the membrane gas may augment diffusion of oxygen across the available surface area into the blood and maintain adequate oxygenation. Increasing the flow rate of gas across the membrane also may help remove condensation and improve gas diffusion. When these methods fail or the pressure difference between entry and exit of blood in the oxygenator continues to rise, the oxygenator may need to be replaced. Although the exact timing for membrane oxygenator failure varies, generally they are not rated for use beyond 7 days. Replacement of the oxygenator is usually a quick and uncomplicated process.
Hollow-Fiber Oxygenator
Hollow-fiber oxygenators are becoming more available for ECMO use and are quickly replacing the silicone oxygenator. These devices are microporous and have long, thin fibers. Blood flows either between the inside or the outside of the fibers. These devices have a lower resistance to blood flow and a lower priming volume than the traditional silicone membrane lung, which makes them faster and easier to prime.50 Early versions of these oxygenators were plagued by plasma leakage difficulties that resulted in very short life spans for the oxygenator and limited their use. Newer versions hold promise as in vivo studies have shown excellent gas exchange, little hemolysis, and durability of days to weeks without failure from plasma leakage. European versions of hollow-fiber oxygenators, now available in the United States, last for weeks at a time.
In one survey, 80 of 103 North American ECMO centers listed in the ELSO directory as neonatal centers (78%) responded to a survey regarding use of different ECMO devices. Of the responding centers, 82.5% routinely used roller pumps for neonatal ECMO, and the remaining 17.5% used centrifugal pumps. Silicone membrane oxygenators were used by 67% of the respondents, whereas 19% used microporous hollow-fiber oxygenators, and 14% used polymethylpentene hollow-fiber oxygenators. A bladder was used by 85% of the centers, and 4% of these used a mechanical bladder box for servoregulation; the remaining 96% used pressure servoregulation. An air bubble detector was used by 88% of the responding centers. A surface coating was used by 44% of the centers on all their neonatal patients treated with ECMO. Thirty-one percent of the centers use an activated clotting time of 180 to 220 seconds.51 It is likely that a survey done today would find an increasing number of centrifugal pumps and hollow-fiber oxygenators being used in many centers.
Other advances in oxygenator development have allowed adequate oxygenation without an intercurrent pump to be incorporated. One device, the Novalung, takes arterial blood from the femoral artery and directs it through a hollow-fiber device for oxygenation and carbon dioxide removal, and then reinfused blood is directed into the femoral vein.52 Experience with this device is growing, and multiple reports of bridging to lung transplantation or to resolution of lung injury can be found in the literature. In addition, an implantable artificial lung is close to being ready for clinical trial.
Other Points
One recent concern with ECMO circuitry is the potential for high levels of Di(2-ethylhexyl)phthalate (DEHP) to be leeched from the polyvinyl tubing over time and thus increase the exposure of the patient to this chemical. One neonatal ECMO circuit evaluation noted that high levels of DHEP were found with routine roller head use over time.53 High levels of DHEP have been shown to cause sterility and endocrine abnormalities in rats. A recent follow-up report of 22 neonatal patients treated with ECMO who are now 14 to 16 years of age found no abnormalities in observed genital development, sex hormone levels, or thyroid, renal, or hepatic function.54 Another study found no accumulation of DEHP in the circulating fluid of ECMO circuits that were pre-primed for up to 30 days prior to clinical use.55
Work with heparin-bonded tubing also has resulted in several commercially available products. Although none has been proved to effectively reduce the need for systemic heparin administration on a long-term basis, short periods (<24 hours) of support without heparin have been achieved without clotting of the circuit or observable thrombosis in the patient. Complete heparin-bonded ECMO circuits that include a hollow-fiber oxygenator are now available and are used especially in situations where bleeding is expected (in postoperative patients, for example) to lessen or avoid the need for anticoagulation. Research into other ways to prevent circuit clotting such as altering platelet aggregation with agents such as nitric oxide also holds promise for the future.56
Patient Populations Treated with Extracorporeal Life Support
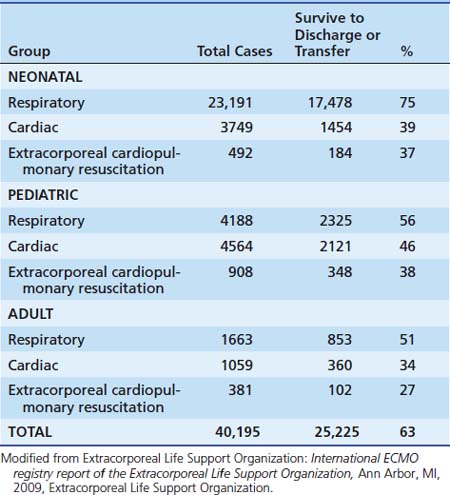
The demographics of patients who have received ECMO support over time is shown in Table 53-1.
Neonatal Cardiopulmonary Failure
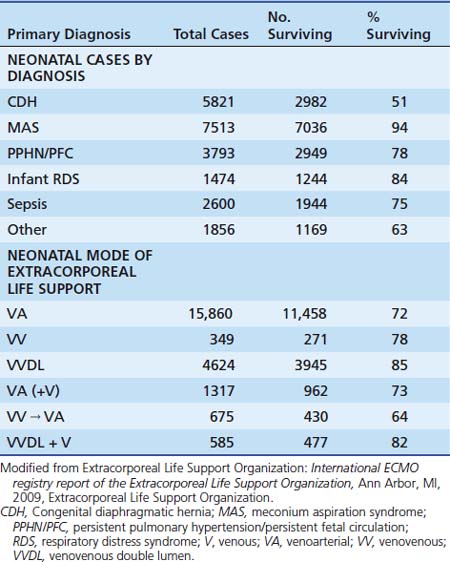
Most patients treated with ECMO have been neonates with severe respiratory failure with an overall survival rate of 75%. Common diagnoses include meconium aspiration syndrome, respiratory distress syndrome, sepsis, and persistent pulmonary hypertension of the newborn (PPHN). With the exception of the last group, most of these infants experience a combination of pulmonary parenchymal and vascular dysfunction that leads to impaired gas exchange.57 The diagnosis, outcome, and mode of ECMO applied in neonatal patients are shown in Table 53-2.
When medical therapies fail to relieve hypoxia and pulmonary hypertension in an infant, ECMO may be an effective means of support. ECMO provides adequate gas exchange and circulatory support without further exposure to high oxygen concentrations or high airway pressures, thus fostering healing of the damaged lungs. The circulatory changes that result from initiation of ECMO also lower pulmonary vascular resistance. Draining right atrial blood reduces right atrial pressure and promotes closure of the foramen ovale. In addition, the reduced blood flow to the pulmonary vascular bed decreases pulmonary flow, reduces pulmonary artery pressure, and relieves right-to-left shunting through the patent ductus arteriosus. Well-oxygenated blood flowing left –to right through the patent ductus arteriosus promotes its closure. By relieving hypoxia, hypercapnia, and acidosis, ECMO promotes relaxation of pulmonary vascular tone. The amount and extent of pulmonary arteriolar smooth muscle begins to regress.58 These changes allow the transition to a mature circulation. The infant continues to receive ECMO until the parenchymal lung disease heals sufficiently to allow adequate gas exchange. However, pulmonary function tests indicate that commonly infants are weaned from ECMO with only moderate improvement in mechanical lung function.59 These observations support the impression that circulatory abnormalities contribute importantly to neonatal “respiratory” failure and may partially explain the dramatic difference in outcome between neonates and older patients treated with ECMO.
Infants with congenital diaphragmatic hernia comprise a special subgroup of patients treated with ECMO for severe pulmonary hypertension and respiratory failure.60,61 Severe pulmonary hypertension or lung hypoplasia leads to about 50% mortality even with ECMO support.62 Morphologic examination, better understanding of the pathophysiology of this lesion, and stabilization with ECMO as needed preoperatively has improved the survival rate in some centers in this challenging group of patients.63–66
As alternative support methods such as high-frequency ventilation (HFV), surfactant, and inhaled nitric oxide (iNO) have been developed and have gained acceptance, the need for ECMO support of neonates has declined.67 Annual neonatal ECMO cases now number around 800 per year, down from the peak of 1500 cases in the early 1990s. Survival rates also have decreased in the neonatal population during the last few years, which is speculated to be related to delays in institution of ECMO while other less invasive therapies are attempted. Infants for whom all other therapies fail and who require ECMO may thus be sicker than in years past when ECMO was the only “rescue” therapy available. While this explanation seems logical, in an evaluation of the ELSO registry data, there was no difference in outcome between infants who received treatment with iNO, HFV, or both prior to ECMO and those who did not (H. J. Dalton and P. Rycus, unpublished data, 2002). Similarly, there was no statistical difference in measures of respiratory severity such as the arterio-alveolar oxygen tension difference (AaDO2) or oxygenation index in the past few years. An alternative explanation is that use of ECMO has expanded from “simple” neonates with respiratory failure to include a variety of neonates with comorbidities that also may influence survival.
Pediatric and Adult Patients
Approximately 250 nonneonatal pediatric patients undergo ECMO each year for severe respiratory failure, with an overall survival rate of 53% (Table 53-3).68 Most of these patients have severe hypoxia, hypercapnia, or intractable air leaks. Pulmonary dysfunction resulting from bacterial or viral pneumonia, aspiration syndromes, intrapulmonary hemorrhage, acute respiratory distress syndrome, and other poorly defined disorders have been treated successfully with ECMO. The uncertainties accompanying the use of ECMO in neonates, who compose a homogeneous group relative to other age groups, are compounded in older children. The enormously heterogeneous older pediatric population spans nearly two decades of physiologic development, and cardiorespiratory failure develops as a result of a multitude of different disorders. Furthermore, many patients have varying degrees of multiple organ failure along with respiratory disease at the time ECMO is instituted. Resolution of both lung disease and secondary organ dysfunction must occur to achieve survival. These factors result in lower survival rates in older patients treated with ECMO than that achieved with the neonatal patient population.69
One small subgroup of persons receiving ECLS support are patients with status asthmaticus. In a recent study comparing results with patients who had asthma and that of the ELSO database, the center reported 13 patients who underwent ECLS between 1986 and 2007.70 The median time from intubation to ECLS was 14 hours (range, 1 to 34 hours). The mean arterial pH was 6.93 (range, 6.78 to 7.03), and PaCO2 was greater than 130 prior to institution of ECLS. Four of the thirteen patients (31%) had a cardiorespiratory arrest in the intensive care unit (ICU) prior to institution of ECLS. Use of medical therapies prior to ECLS included intravenous β-agonist in 100%, ketamine infusion in 92%, administration of magnesium sulfate in 69%, helium/oxygen in 69%, and use of an anesthetic inhalational agent in 62%. The mean duration of ECLS was 95 hours (range, 42 to 395 hours). The survival rate was 100%, with no neurologic sequelae noted. Venovenous cannulation was done in 95% of the patients. A second asthma group can be found in the ELSO database, with a total of 64 patients supported by ECLS over the same time frame (including the aforementioned 13 patients). There was little difference in the pre-ECLS variables of age, PaCO2, and pH between the groups. The mean duration of ECMO was 94 hours, and 94% of the patients survived. Venovenous cannulation was done in 86% of patients.70
Despite attempts to define predictive models for ECMO candidacy and institution, none have proved universally applicable. The clinician still most often relies on clinical judgment at the bedside when determining when conventional medical therapy has failed. Perhaps the largest change in nonneonatal ECMO in the past few years is the variety of patients to whom it has been applied. Patients with recent trauma, tracheal injury requiring reconstruction, burns, smoke inhalation, and severe sepsis, along with those who are immunocompromised, and those who have had a toxic ingestion with cardiopulmonary collapse, are but a few of the types of patients who might have been excluded from ECMO support a few years ago but have now received successful ECMO therapy.71–74 The multiple exclusion criteria used in the early days of ECMO have now been fairly well eliminated, and each potential patient is generally considered on a case-by-case basis. Even patients with known bleeding disorders such as hemophilia have successfully received ECMO support.75
In a recent review of the ELSO data registry, 107 patients with malignancy and respiratory and/or cardiac failure were analyzed. Seventy percent of patients had hematologic disease and 30% had solid tumor malignancy; 80% of the patients required ECLS primarily for pulmonary support. Forty-two percent of patients were weaned off ECLS support, with 35% surviving to hospital discharge. Risk factors for death in the pre-ECMO period were a lower PaO2, higher oxygenation index, and a higher level of positive end-expiratory pressure (PEEP). Development of renal or cardiopulmonary complications during ECMO also was associated with poor outcome. Deaths were attributed to irreversible organ failure (34%), diagnosis incompatible with life (10%), hemorrhage (5%), and family request to withdraw support (7%). No difference in outcomes was noted in patients with either hematologic malignancies or solid tumors.76
Although initiation of ECMO support prior to the development of multiple organ system failure is desired, many patients present with established organ failure already in progress. ECMO may provide an optimal environment for organ recovery in such patients, not just providing respiratory or cardiac support. Further, the ability to add adjunctive devices such as hemofiltration or dialysis (Figure 53-6) to augment renal function and allow use of plasmapheresis or plasma exchange or hepatic support devices is another aspect of ECMO support that may facilitate patient care and organ recovery. Implementation of ECMO during multiple organ systems failure now occurs under a variety of conditions, with resolution of organ dysfunction and good outcome often obtained.77 Furthermore, because it avoids the circulatory derangements that often result from extreme forms of mechanical ventilation and provides systemic perfusion without the need for high-dose levels of inotropic agents, ECMO also may prevent damage to other systems.
Figure 53–6 Continuous renal replacement therapy in use with extracorporeal membrane oxygenation circuit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree