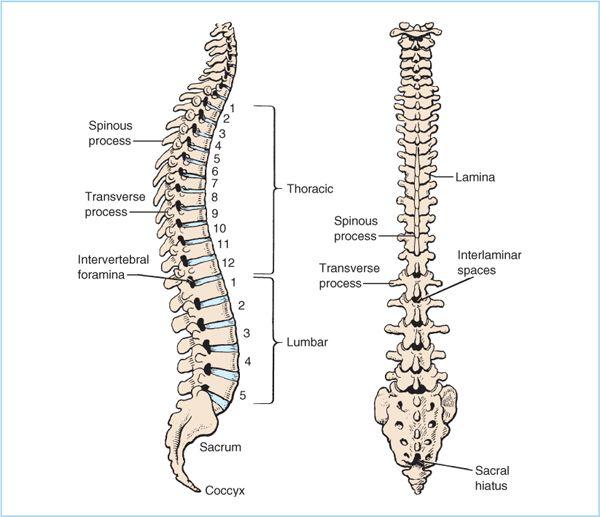
Figure 7.1. Anatomy of the bony and ligamentous components of the lumbar spine. The same structures are present at all vertebral levels although the shapes of the structures differ with vertebral level, as do the relationships between the bony components and the interlaminar space. Note also the location of the epidural veins in the anterior epidural space.
C. Epidural fat. Epidural fat lies between the dura mater and the vertebral canal. Although often incorrectly depicted as a continuous, uniform sheet surrounding the spinal cord, Hogan has shown that the epidural fat actually lies in discrete pockets in the posterior and anterolateral epidural space (Figure 7.3). The posterior fat compartment separates the ligamentum flavum from the dura mater, and thereby helps prevent the epidural needle from entering the subarachnoid space as it exits the ligamentum flavum.
1. Because hydrophobic drugs can be extensively sequestered in epidural fat, it plays an important role in their pharmacokinetics (2). Whether fat acts as a reservoir that prolongs block duration or as a sink that decreases the amount of available drug (thereby slowing onset) or both is unclear.
D. Epidural veins. Although epidural veins are often portrayed as a reticular network surrounding the spinal cord, this view of the epidural venous plexus (Bateson plexus) is incorrect. Epidural veins are almost always confined to the anterior epidural space and only very rarely does a vein reside posterior to the intervertebral foramen through which the spinal nerves exit (3,4).
E. Dura mater. The dura mater, which is composed almost entirely of randomly oriented collagen fibers (5), forms the inner limit of the epidural space. The “dural sac” tapers to an end at approximately L5 where it continues through the sacral vertebral canal as the filum terminale. Consequently, the volume of the epidural space is larger below L5, a fact that likely explains the large volume of local anesthetic necessary to extend caudal epidural anesthesia to low thoracic levels. Anteriorly, the dura mater frequently fuses with the posterior longitudinal ligament thereby “obliterating” the anterior epidural space and preventing fluid spreading across the midline anteriorly.
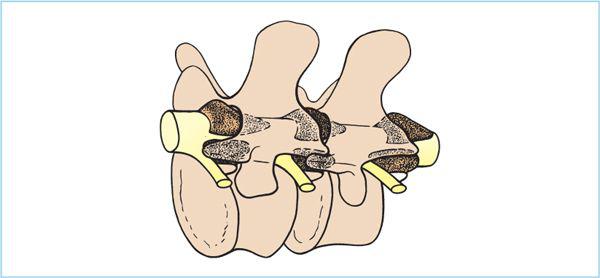
Figure 7.2. Comparative lumbar and thoracic spinal anatomy.
III. Pharmacology
A. Site of action. The precise site of action of epidurally administered local anesthetics is not known. Studies in humans and animals indicate that local anesthetics penetrate the spinal meninges to reach the cerebrospinal fluid (CSF) in concentrations comparable to those produced during spinal anesthesia. However, spinal cord transmission remains intact indicating that the spinal cord itself is not the site of action. Animal studies demonstrate relatively high and comparable local anesthetic concentrations in both extradural spinal nerves traversing the epidural space and in spinal nerve rootlets within the subarachnoid space. It is not known which of these sites is the principal site of action, but is not unreasonable to assume that both play a role.
Figure 7.3. Epidural fat (stippled area) is discontinuously distributed within the epidural space. In areas where fat is absent, the dura mater abuts the ligamentum flavum and represents a “potential space.” (Adapted from Hogan Q. Lumbar epidural anatomy: a new look by cryomicrotome section. Anesthesiology 1991;75:767.)
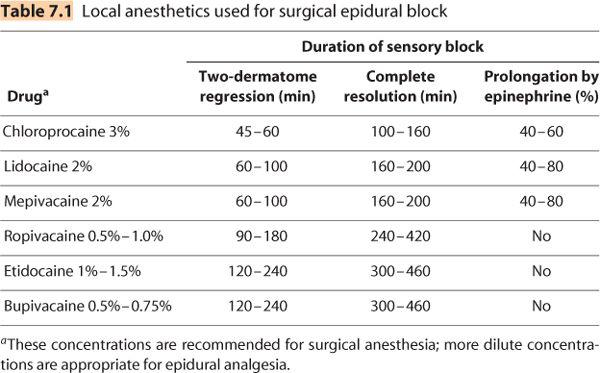
B. Local anesthetic drugs. Nearly all local anesthetics have been used for epidural anesthesia. Because of concerns about neurotoxicity only preservative-free local anesthetic solutions should be used in the epidural space. Local anesthetics are commonly organized in terms of their duration of action. However, the “duration” of any block varies depending on how “duration” is defined. For epidural anesthesia, “two-dermatome regression” is often used and is defined as the amount of time it takes a block to recede by two dermatomes from its maximum extent. Two-dermatome regression is a reasonable estimate of the duration of effective surgical block. Complete resolution is the time it takes for the patient to recover completely from sensory block and is a reasonable estimate to use for the time until outpatients may be ready for discharge. Table 7.1 lists both durations. Drugs currently used for epidural anesthesia are listed subsequently.
1. Short duration
a. Chloroprocaine (2% or 3%) is currently available as a preservative-free solution for epidural anesthesia. Chloroprocaine produces the fastest onset and the shortest duration epidural block (Figure 7.4), although duration can be extended indefinitely by using an epidural catheter. Use of large chloroprocaine doses (more than 1,200 mg) and the presence of ethylenediamine tetraacetic acid (EDTA) have been associated with postepidural back pain more than other local anesthetics in some studies (6). Back pain has also been reported following large epidural doses of preservative-free chloroprocaine (3,000 mg over more than 7 hours) (7), whereas studies using more modest doses (900 mg) have found only mild back pain, which was not different than that experienced by subjects receiving lidocaine (8). Epidural chloroprocaine has also been associated with reduced efficacy of subsequently administered epidural morphine (9) and epidural clonidine (10) for reasons that are not known.
b. Procaine is not a reliable epidural local anesthetic.
2. Intermediate duration. These drugs produce a rate of block onset that is not much different than chloroprocaine, but they have a slower rate of resolution, which may result in delayed discharge of ambulatory patients.
a. Lidocaine (1.5% or 2.0%) produces excellent anesthesia of 60- to 90-minute duration as a single injection, but has been associated with tachyphylaxis (decreasing duration with repeated injection) when repeatedly administered through an epidural catheter. The mechanism is not known but appears not to be the result of changes in drug distribution within, or elimination from, the epidural space (11).
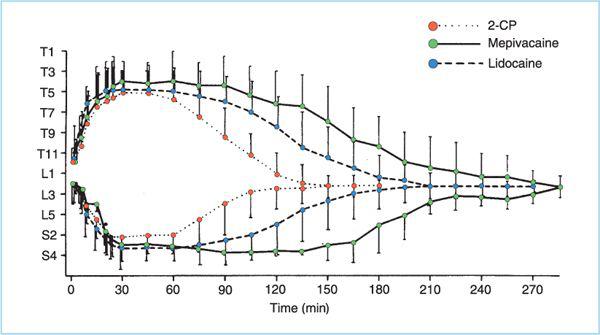
Figure 7.4. Onset and duration of epidural anesthesia. Sensory dermatomal blockade level (with standard deviations) versus time following injection of 20 mL of 3% 2-chloroprocaine (CP), 1.5% lidocaine, or 1.5% mepivacaine with 1:200,000 epinephrine at the L2 interspace. Average total durations were 133, 182, and 247 minutes, respectively. (Adapted from Kopacz DJ, Mulroy MF. Chloroprocaine and lidocaine decrease hospital stay and admission rate after outpatient epidural anesthesia. Reg Anesth 1990;15:19, with permission.)
b. Mepivacaine (1% or 1.5%) produces a somewhat longer average block than lidocaine.
3. Long duration. Given the ease with which the epidural space is catheterized, the long duration of these drugs is less of an advantage and can be a significant disadvantage for outpatient epidural anesthesia in which rapid recovery is important.
a. Bupivacaine (0.5% or 0.75%) is supplied as a racemic mixture of the levoand dextrorotary optical isomers. It is reported to produce a somewhat denser sensory block compared to motor block, which has made it a favored drug for epidural analgesia (especially dilute concentrations). It also has slower uptake from the epidural space than the intermediate-duration local anesthetics, and therefore has less potential for systemic toxicity caused by local anesthetic absorption. Because of bupivacaine’s cardiovascular toxicity (see Chapter 3) it is important to avoid high doses and avoid intravascular injection by appropriate application of a test dose use.
b. Levobupivacaine, the levorotary isomer of bupivacaine, is essentially indistinguishable from the racemic mixture in every way except that it is less cardiotoxic. Levobupivacaine is currently not available in the United States.
c. Ropivacaine is a single optical isomer that is approximately 40% less potent than bupivacaine in the epidural space. If one accounts for the potency difference, it is not significantly less cardiotoxic than bupivacaine nor does it have significantly greater “motor sparing” effects than equipotent concentrations of bupivacaine. However, it is more expensive.
d. Although etidocaine produces effective and long-lasting epidural block, its use has largely been abandoned because of an unusual tendency to produce relatively longer duration motor block than sensory block, and it is currently not available in the United States.
C. Adjuvants. The duration of sensory and/or motor block produced by different local anesthetics can be “fine-tuned” by addition of a variety of adjuvants, including:
1. Epinephrine
a. Block prolongation. Epinephrine at a concentration 5 μg/mL (1:200,000 mg/mL) prolongs the duration of both sensory and motor block produced by short- and intermediate-duration local anesthetics, but not long-duration drugs. The mechanism(s) by which the block is prolonged is not precisely known.
(1) Evidence of a pharmacokinetic mechanism comes from human studies showing that addition of epinephrine decreases peak plasma concentration, which suggests slower drug clearance from the epidural space. This has been confirmed in animal studies (12). Contrary to what has often been taught, epinephrine does not decrease clearance by constricting the epidural venous plexus. Rather, animal studies showing that epinephrine decreases blood flow in the dura mater (13) suggest that this is the mechanism by which epinephrine slows local anesthetic clearance.
(2) In addition to a pharmacokinetic effect, epinephrine may also have a pharmacodynamic effect. Specifically, because epinephrine is an α2-adrenergic agonist it may also act within the spinal cord to decrease pain transmission. The ability of epinephrine to improve postoperative analgesia when added to dilute concentrations of epidural bupivacaine may be evidence of this mechanism.
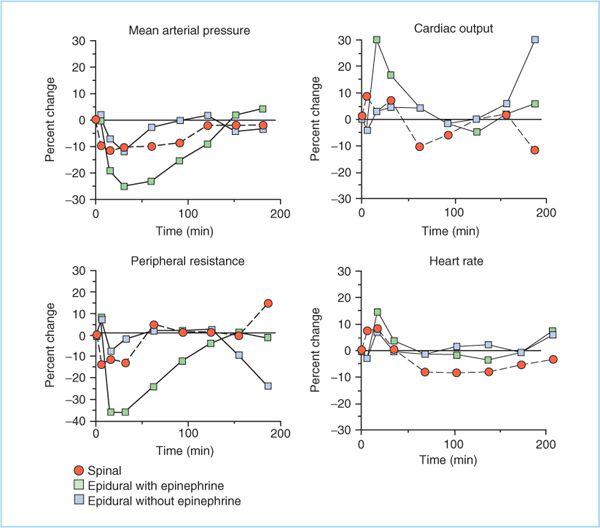
b. Hemodynamic effects. Compared with plain local anesthetics, addition of epinephrine to epidural block results in a markedly greater decrease in mean arterial pressure (MAP) (Figure 7.5) (14). The decrease in MAP is caused by a greater fall in systemic vascular resistance (SVR), presumably because of the vasodilatory β2-adrenergic effects of low dose epinephrine. The decrease in SVR also results in a significantly higher cardiac output than occurs with epidural block without added epinephrine. Heart rate is modestly higher when epinephrine is added. Whether this is a direct effect of epinephrine or a reflexive response to decreased MAP is unknown. Animal studies indicate that the presence of epinephrine in the local anesthetic solution does not decrease the risk of cardiovascular toxicity in the event of accidental intravascular injection (15).
Figure 7.5. The cardiovascular effects of spinal and epidural anesthesia in volunteers with T5 blocks. The effects of spinal anesthesia and epidural anesthesia without epinephrine were generally comparable and are both qualitatively and quantitatively different from the effects of epidural anesthesia with epinephrine. (Adapted from Bonica JJ, Kennedu WF Jr, Ward RJ, et al. A comparison of the effects of high subarachnoid and epidural anesthesia. Acta Anaesthesiol Scand Suppl 1996;23:429.)
2. Opioids. Addition of opioids to epidural local anesthetics increases the duration of sensory, but not motor block. The magnitude and duration of the effect depends on the opioid chosen (hydrophobic opioids are significantly shorter than hydrophilic opioids) and the dose administered.
3. Clonidine. Epidural clonidine (150–300 μg) prolongs sensory, but not motor block and unlike epinephrine the effect occurs with long-acting local anesthetics (16,17). Epidural clonidine is rapidly cleared into plasma and redistribution to brain sites (locus coeruleus) causes sedation. Clonidine also causes decrease in blood pressure, likely mediated through spinal, brain and peripheral α2-adrenergic actions. However, the effect on blood pressure is less than that of epinephrine (16). Unlike epinephrine, epidural clonidine is associated with a modest decrease in heart rate (16).
4. Bicarbonate. Addition of sodium bicarbonate (0.1 mEq/mL) to epidural local anesthetics has been advocated as a means to speed the onset of epidural block. However, published studies are nearly evenly split between those that found a faster onset with bicarbonate and those that found no difference. This is true for lidocaine, mepivacaine, chloroprocaine, and bupivacaine. At best, bicarbonate would seem to have an unreliable effect on block onset.
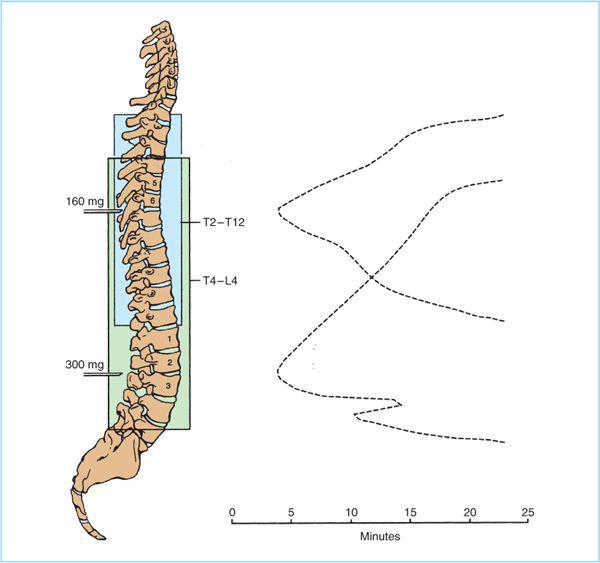
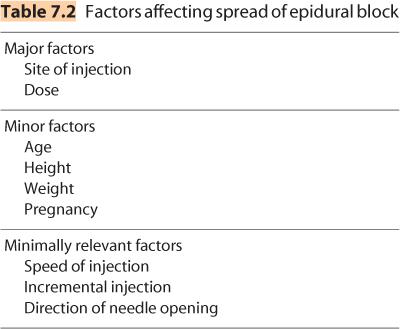
D. Dose. Within the epidural space, local anesthetic solutions spread cephalad and caudad from their injection site and produce a band of anesthesia that correlates reasonably well with the extent of solution spread (Figure 7.6). Unfortunately, it is impossible to look at any individual patient and predict with certainty what dose of local anesthetic is necessary to produce a given extent of epidural blockade. Consequently, clinicians must be aware of the major and minor factors that contribute to determining the spread of epidural block (Table 7.2) and use this information in conjunction with knowledge of the dermatomes that must be blocked for a particular surgical procedure to decide the local anesthetic dose necessary.
1. Dose, volume, and concentration. Both drug dose and volume are independent predictors of the spread of epidural blockade. That is, increasing drug dose while holding volume constant (increasing drug concentration) will increase the extent of epidural blockade. Conversely, increasing drug volume while holding dose constant (decreasing concentration) will also increase the extent of epidural blockade. However, the relationship is not linear; as dose is increased the spread per milliliter injected decreases such that the net effect is only a few dermatomes increase in spread.
2. Technique
a. Location. Because local anesthetic spreads cephalad and caudad form the site of injection, the injection site is a major determinant of which dermatomes will be blocked by a given local anesthetic dose. In addition, the volume of the epidural space increases as one moves caudad; consequently to anesthetize the same number of dermatomes may take 25 mL in the caudal epidural space but only 8 mL in the thoracic epidural space.
Figure 7.6. Diagram of spread of local anesthetic in the epidural space. The onset of epidural anesthesia is noted first in the segments nearest the site of injection, and spreads over the next 20 minutes both cephalad and caudad from this point.
b. Patient position. Gravity has no clinically important effect on the spread of local anesthetics in the epidural space.
c. Needle angle/aperture direction. Paramedian and midline needle insertion produce the same local anesthetic spread. Turning the needle aperture to face cephalad or caudad produces slightly greater spread in the direction the aperture faces. The magnitude of the effect is not clinically important.
d. Injection speed. Speed of local anesthetic injection has a very minor effect on spread of epidural block (faster = farther).
e. If the catheter lies in the midline, then spread should not differ significantly from that which would occur through a needle. However, catheters have an annoying tendency to deviate significantly from the midline and if they end up traversing a vertebral foramen or lying very anterior in the epidural space then spread may be reduced and/or asymmetric (i.e., unilateral). This problem may be reduced by putting less catheter in the epidural space (3–4 cm) and using larger local anesthetic volumes. Malposition of the catheter may be particularly problematic when it is used for postoperative analgesia because of the relatively low volumes of dilute local anesthetic that are typically used.
3. Patient factors
a. Gender is not an important determinant of local anesthetic spread.
b. Studies of the effect of pregnancy are conflicting with some studies finding greater spread at all stages of pregnancy and others finding no difference. Interestingly, pregnant women have been shown to be more sensitive to the blocking effects of local anesthetics, which would be consistent with greater epidural spread.
c. On average, increasing age results in an increase in the spread of epidural anesthesia, but the magnitude of the effect is not as great as once thought (18,19). The difficulty in using age as a factor in choosing a dose is that the interindividual variability is so great that it is impossible to predict a priori how age will affect block height in any individual patient.
d. Height. On average, spread of epidural block is greater in shorter people. But, as with age, the interindividual variability is so great that it is difficult to predict how height will affect epidural block in any individual patient.
e. Weight. On average, obesity increases the spread of epidural block. But, like age and height, the effect is small and highly variable among individuals.
4. Choosing a dose. As can be deduced from the preceding, the choice of local anesthetic dose is highly subjective. One approach is to consider 15 mL as an average starting dose for lumbar injections. If multiple factors suggest a reason that a larger dose may be necessary (e.g., large spread needed for planned surgery, patients who are very young and tall) then increase the dose by 5 to 10 mL. Conversely, if multiple factors suggest a reason to reduce the dose (e.g., small spread needed for planned surgery, patients who are unusually short, obese, or old) then reduce the dose by 3 to 5 mL. For thoracic epidural blocks a reasonable average starting volume would be 6 to 8 mL. This dose might be increased by 2 to 6 mL or decreased by 1 to 2 mL for reasons outlined earlier. Importantly, it is often easier to deal with a block that is more extensive and longer lasting than necessary than it is to cover up for a block that is inadequate. Of course, use of an epidural catheter technique renders this problem moot because it permits “titration” of the epidural block.
IV. Technique
Epidural block can be performed with the patient in any position that permits access to the back (prone, lateral, sitting), although lateral is the most common and often the most comfortable for the patient. Sitting can be advantageous in the morbidly obese patient because it is easier to identify the midline. In the lumbar, low thoracic, and cervical spine, epidural blockade is similar to spinal anesthesia and is generally performed in the midline. In the high- through midthoracic spine the paramedian approach is usually necessary. As with any regional anesthesia procedure, equipment for monitoring, resuscitation, and treating side effects must be immediately available.
A. Midline. For the midline approach the following steps are taken:
1. Prepare equipment. Ideally, prepare the epidural “tray” before positioning the patient. Draw up local anesthetic, fill “loss-of-resistance syringe,” and uncap needles, and so on. Doing this “prep-work” before positioning the patient will minimize the amount of time the the patient has to remain in a relatively uncomfortable position.
2. Sedate patient as deemed appropriate (see Chapter 4). Because the midline approach does not make use of intentional contact with bone/periosteum, it should not be very painful.
3. Position patient laterally with knees drawn up so that the legs are maximally flexed at the hips. Instruct patient to “curl up like a boiled shrimp” or similar visual metaphor. Place patient at the edge of the bed (Beware. Do not walk away from a patient thus positioned because of the risk that he or she will roll backwards onto the floor) with hips and shoulders perpendicular to the bed. This position will maximize distraction of spinous processes and minimize the need to lean over and reach for the patient.
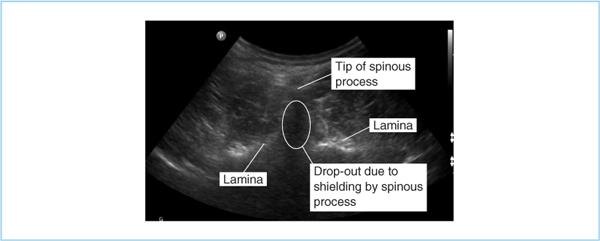
4. Locate desired interspace using the iliac crest as a landmark to locate L4 (a line through the iliac crests crosses L4 ± 1 vertebral body; in obese patients the fat overlying the iliac crests may bias your estimate in a cephalad direction.) Marking the intended space with a skin pen may reduce the amount of time spent reidentifying the space throughout the procedure. Finding spinous processes can be difficult in the morbidly obese. A 90-mm (3.5-in.) spinal needle is sometimes useful as a finder needle to identify bony landmarks. Additionally, ultrasonography can sometimes be used to identify spinous processes in obese patients (Figure 7.7). Unfortunately, the limited resolution that can be achieved with the low frequencies necessary to reach greater tissue depth limit the ability to use ultrasonography in very obese subjects—the very subjects in whom it would be most useful.
5. Prepare the skin with appropriate antiseptic and drape as for spinal anesthesia. Use of clear plastic drapes makes it easier to reevaluate landmarks and reposition patient position if necessary.
6. Anesthetize skin, subcutaneous tissue, and a track along the intended path of the epidural needle. In this way the needle can be used as a “finder needle” to delineate the path between the spinous processes. Take care not to deposit a large volume of local anesthetic in the subcutaneous tissue because the resultant “mound” may make it difficult to feel the interspace, especially in obese patients.
Figure 7.7. Ultrasound image of lumbar spine. The “drop-out” below the spinous process is caused by the inability of ultrasound waves to penetrate the bone. Laminas are clearly identified as bright hyperechoic areas.
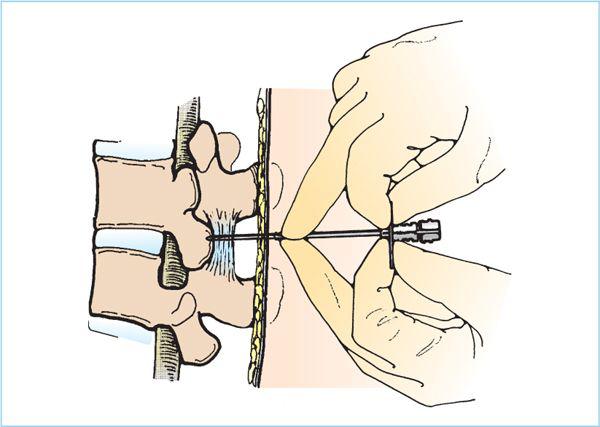
7. Insert the epidural needle through the skin with the bevel of a Tuohy or Hustead needle oriented cephalad or caudad. Orienting the bevel laterally may cause the needle to deviate from the midline. Insertion should proceed slowly and under control at all times (Figure 7.8). Passage through the interspinous ligament often results in a “gritty” sensation as if pushing the needle through a bag of tightly packed sand. Failure to appreciate this grittiness should alert one to the possibility that the needle is offmidline and not within the interspinous ligament. As the needle enters the ligamentum flavum, resistance will increase subtly; stop at this point. The depth to the ligamentum flavum is generally between 3.5 and 5 cm in normal-sized adults, but may be significantly deeper in the obese.
The ability to detect the increased resistance of the ligamentum flavum is an acquired skill, and it is not uncommon for the novice to have several unintentional meningeal punctures before mastering this step. Pregnant women are notorious for having “soft” ligamenta flava, and it can be particularly difficult to correctly identify the ligament in this group.
8. After identifying the ligamentum flavum, remove the stylet of the needle and attach a 5- to 10-mL saline-filled syringe containing a clearly visible air bubble (0.1–0.5 mL). The air bubble will act as a gauge of the amount of pressure exerted on the syringe barrel (see subsequent text). Freely moving glass syringes give a better loss of resistance than plastic syringes, although specially designed low-resistance plastic syringes intended for epidural anesthesia are also appropriate. Care must be taken to ensure that the glass syringe barrel does in fact move freely; “sticky” syringes make it very difficult, if not impossible, to detect a loss of resistance. Position the back of the nondominant hand against the patient’s back and grasp the epidural needle with the thumb and forefinger (Figure 7.9). This hand is used to control needle movement and prevent accidentally plunging into the subarachnoid space. Apply continuous pressure on the syringe plunger with the thumb of the dominant hand. If the needle tip is in the ligamentum flavum it should be possible to exert enough pressure on the plunger to visibly compress the air bubble without injecting fluid. If it is not possible to compress the air bubble without injecting fluid then the needle tip is probably not in the ligamentum flavum. Advance the needle slightly and reattempt compression. Repeat as necessary until compression is achieved. If compression does not occur at a “reasonable depth” consider that the needle may be off the midline and reevaluate landmarks/needle angle.
Figure 7.8. Hand position for epidural needle insertion into the ligamentum flavum. The epidural needle must be inserted under control at all times to prevent deviation from the intended path or a sudden “plunge” into the subarachnoid space or spinal cord. Multiple hand positions are appropriate, but the technique depicted here is widely employed. The thumb and forefingers hold the flanges extending from the needle hub and the tips of the middle fingers rest on the back and “grasp” the needle shaft. Resting the fingers on the back prevents the needle from being unintentionally advanced should the patient move unexpectedly. The needle is advanced using the thumbs, forefingers, and wrists—not the arms; this permits controlled advancement and appreciation of the subtle increase in resistance that heralds entry into the ligamentum flavum.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree