INTRODUCTION
The authors acknowledge the special contributions of Peter Hackett, MD, The Institute for Altitude Medicine Telluride, CO; Edward Otten, MD, University of Cincinnati, Cincinnati, OH; James O’Malley, MD, Providence Alaska Medical Center, Anchorage, Alaska; Murray Hamlet, DVM, and Kathy McCue, MD, Alaska Native Medical Center, Anchorage, Alaska; Sheryl Olson, RN, Manitou Springs, CO; Luanne Freer, MD, Yellowstone National Park, The Nashville Zoo, Nashville, TN; and the Nova Scotia Museum of Natural History, Halifax, Nova Scotia, Canada. The authors thank Joseph C. Schmidt, MD, Lawrence B. Stack, MD, and Alan B. Storrow, MD, for their contributions to prior editions.
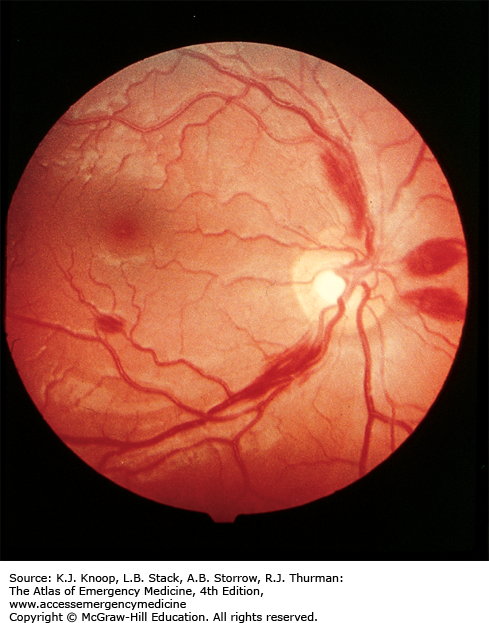
HIGH-ALTITUDE RETINAL HEMORRHAGE
Retinal hemorrhages are common above 5200 m (17,000 ft) and are not always associated with acute mountain sickness (AMS). High-altitude retinal hemorrhages (HARH) are rarely symptomatic, but if found over the macula, these hemorrhages may cause temporary blindness. The diagnosis can be established by ophthalmoscopy. Without visualization of the lesion, the differential diagnosis of unilaterally decreased vision or blindness at high altitude includes migraine equivalent, cerebrovascular accident, and dry eye (often unilateral, due to strong winds), as well as all conditions found at sea level.
HARH generally resolve spontaneously after descent to lower altitudes. No treatment is necessary for asymptomatic HARH. Patients with HARH associated with a decrease in vision should be referred to an ophthalmologist for follow-up.
Patients with blurred vision and unilateral mydriasis at the high altitude should be asked about use of medications, including transdermal scopolamine patches.
As with almost all altitude-related problems, descent is the primary treatment. This is not emergent unless associated with severe altitude illness or progressive visual loss.
Although most symptomatic HARH resolve completely in 2 to 8 weeks, cases of permanent paracentral scotomata have been reported.
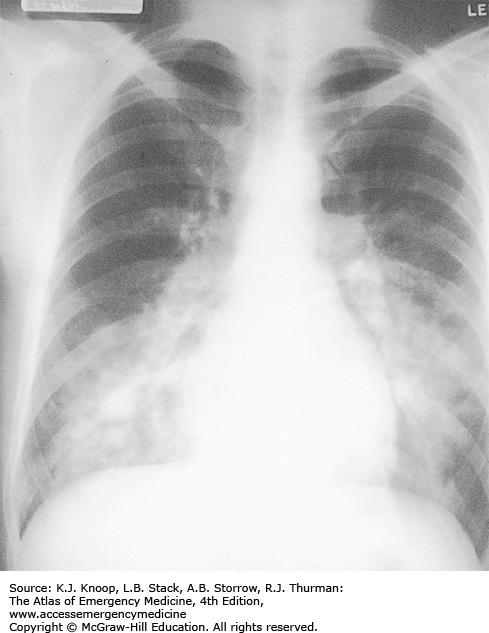
HIGH-ALTITUDE PULMONARY EDEMA
High-altitude pulmonary edema (HAPE) is a form of noncardiogenic pulmonary edema, generally beginning within the first 2 to 4 days after ascent above 2500 m (8200 ft). The earliest symptoms are fatigue, weakness, dyspnea on exertion, and decreased exercise performance. Symptoms of Acute mountain sickness (AMS) such as headache, anorexia, and lassitude may also be present, but HAPE may develop without AMS. First symptoms usually include persistent dry cough and dyspnea followed by tachycardia, tachypnea, and cyanosis at rest. Patients suffering from HAPE often experience sudden onset of symptoms upon awakening after the second night at altitude. Eventually the victim develops dyspnea at rest and orthopnea with audible crackles in the chest. Pink frothy sputum is a grave sign. Patients may experience concurrent mental status changes and ataxia due to hypoxemia or associated high-altitude cerebral edema (HACE).
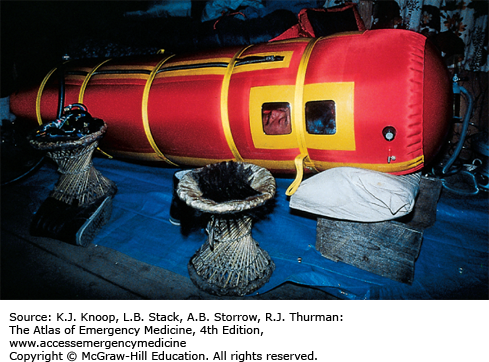
Mild cases (oxygen saturation in the 90s on low-flow oxygen) at moderate altitudes (below 3500 m, 11,500 ft) may be treated at altitude with bed rest and oxygen. If supplemental oxygen and a reliable person are available, the patient may be discharged with oxygen therapy and bed rest at home or in lodgings. Patients with more severe cases should descend immediately. These patients may require admission to a hospital at a lower altitude and, in extreme cases, intubation and mechanical ventilation. Nifedipine is of benefit but is not a substitute for descent. Some experts now use PDE-5 inhibitors such as sildenafil or tadalafil instead of nifedipine. Hyperbaric therapy, especially with a portable hyperbaric chamber (Gamow bag), has an efficacy equal to that of supplemental oxygen and is mainly helpful in prehospital settings where oxygen availability is limited.
Crackles may be unilateral or bilateral but usually start in the right middle lobe and are heard first in the right axilla.
HAPE limited to the left lung in association with a small right hemothorax without pulmonary markings is pathognomonic for unilateral absent pulmonary artery syndrome. These patients develop HAPE at relatively low altitudes, sometimes below 2500 m.
Patients treated for HAPE may resume normal activities once they are asymptomatic and may ascend further during the same trip.
HIGH-ALTITUDE CEREBRAL EDEMA
Acute mountain sickness (AMS) is a symptom complex which usually begins 12 to 24 hours after ascent to high altitude and consists of headache and one or more other symptoms, including gastrointestinal symptoms, fatigue and/or weakness, dizziness and/or lightheadedness, and difficulty sleeping. High altitude cerebral edema (HACE) is a severe form of AMS, clinically defined by the presence of acute truncal ataxia, altered mental status, or both. Usually this occurs as a progression from AMS to HACE, but HACE may occur without antecedent AMS. If not effectively treated, HACE may progress to coma or death. Focal neurologic findings other than truncal ataxia are rare and should suggest another diagnosis, such as acute stroke or venous sinus thrombosis.
Treatment of HACE consists of immediate descent or evacuation, oxygen, and high-dose dexamethasone. Simulated descent using a portable hyperbaric bag (Gamow bag) may be more effective than oxygen alone and may be used in place of oxygen in field settings. Actual descent may be complicated by the inability of the patient to walk unassisted or at all. Patients with HACE may not ascend during the same trip and probably should not reascend to altitude for several months.
FIGURE 16.4
HACE-Related Ataxia. Ataxia due to HACE in the Nepal Himalayas. The patient (middle) developed HAPE and severe truncal ataxia at 5600 m (18,400 ft). The patient required full ambulatory assistance. Descent was started immediately. With descent, the patient’s breathing improved, but he remained too ataxic to walk. (Photo contributor: Ken Zafren, MD.)
The first sign of HACE is usually truncal ataxia. This should be tested using tandem gait (heel-to-toe walking).
HACE may occur without symptoms of AMS.
HACE is often associated with some degree of HAPE.
HYPOTHERMIA
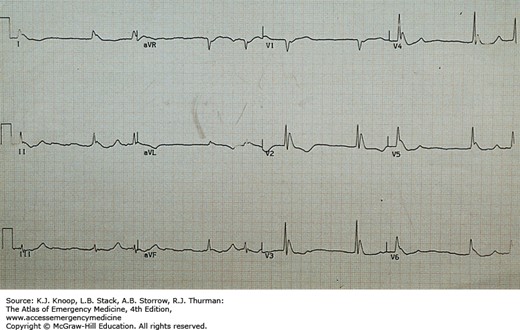
Accidental hypothermia is an unintentional decline in core temperature below 35°C (95°F). Presentation may be obvious or subtle, especially in urban settings. Symptoms vary from vague complaints to altered levels of consciousness. Physical findings include progressive abnormalities of every organ system. Following initial tachycardia, there is progressive bradycardia (50% decrease in heart rate at 28°C [82.4°F]) with decline in blood pressure and cardiac output. ECG intervals are prolonged, beginning with the PR interval followed by the QRS interval and finally the QT interval. A J wave (Osborn wave; hypothermic “hump”) may be seen, but is neither pathognomonic nor prognostic. The J wave is present at the junction of the QRS complex and the ST segment. J waves may also be associated with central nervous system lesions, focal cardiac ischemia, young age, and sepsis. In mildly hypothermic patients, invisible preshivering muscle tone may obscure P waves.
Core temperature measurement is best made with an esophageal probe inserted into the lower third of the esophagus. Rectal temperature is less accurate without the use of a special low-reading thermometer. Gentle handling and appropriate warming methods are the mainstays of emergency department (ED) treatment. Cardiovascular instability often complicates rewarming; Advanced Cardiac Life Support (ACLS) guidelines for hypothermia provide guidance. If not obvious, a precipitating cause should be sought (eg, hypothyroidism, hypoglycemia, sepsis), as should associated pathology. Most patients require admission for observation or to treat associated injuries or comorbidities.
The most common problem with the misdiagnosis of hypothermia in the ED stems from incomplete data on vital signs.
Accurate core temperatures, preferably by esophageal probe, and continuous cardiac monitoring are crucial to appropriate management.
Atrial arrhythmias are generally benign and should not be treated. They resolve with rewarming.
Most cardiovascular drugs are inactive during hypothermia and should be given only once the body temperature is above 35°C (95°F).
FIGURE 16.6
J Waves. J waves in a hypothermic patient with core temperature (rectal probe) of 25.5°C. J waves may be seen at any temperature below 32.2°C, most frequently in leads II and V6. Below a core temperature of 25°C, they are most commonly found in the precordial leads (especially V3 and V4) and their size increases. J waves are usually upright in aVL, aVF, and the left precordial leads (see also Fig. 23.44A “Hypothermia with Osborne Waves (“J” Waves) Present”). (Photo contributor: Alan B. Storrow, MD.)
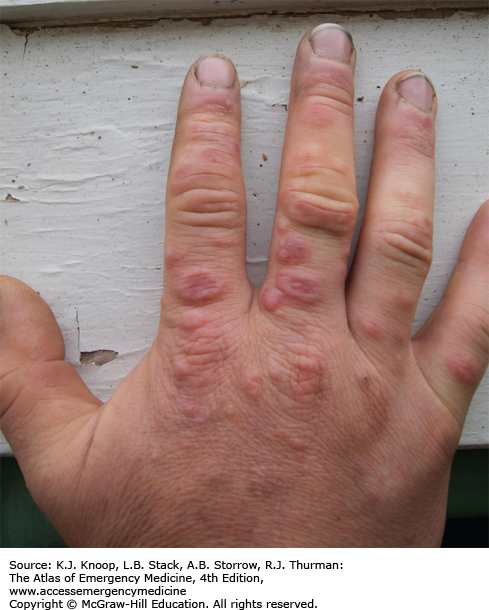
FROSTBITE
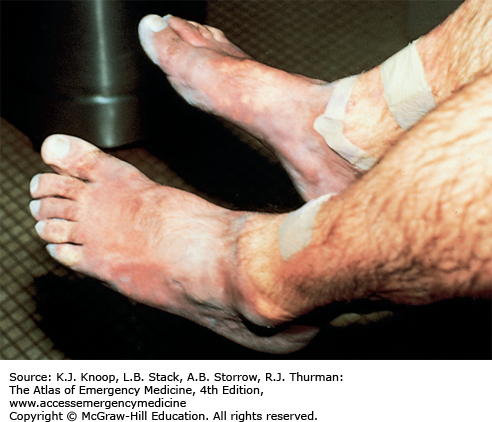
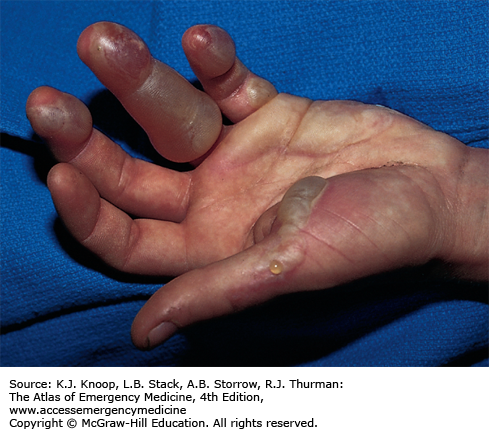
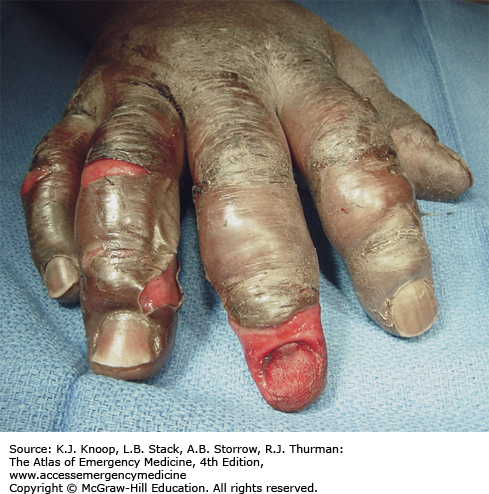
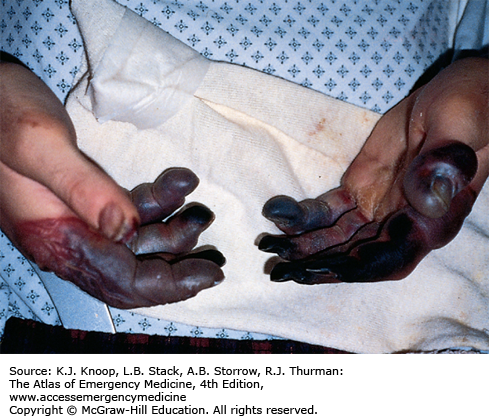
Frostbite is tissue freezing resulting from heat loss sufficient to cause ice crystal formation in superficial or deep tissue. Frostbite usually affects the extremities, nose, or ears (and the scrotum and penis in joggers). A sensation of numbness with accompanying sensory loss is the most common initial complaint. Often, by the time the patient arrives in the ED, the frozen tissue has thawed. The initial appearance of the overlying skin may be deceptively benign. Frozen tissue may appear mottled blue, violaceous, yellowish-white, or waxy. Following rapid rewarming, there is early hyperemia even in severe cases.
Favorable signs include return of normal sensation, color, and warmth. Edema should appear within 3 hours of thawing; lack of edema is an unfavorable sign. Vesicles and bullae appear in 6 to 24 hours. Early formation of large clear blebs that extend to the tips of affected digits is a good indicator. Small dark blebs that do not extend to the tips indicate damage to subdermal plexi and are a poor prognostic sign. When seen early or after rewarming occurs, frostbite may be indistinguishable from nonfreezing cold injury such as immersion foot. Mixed injuries are common.
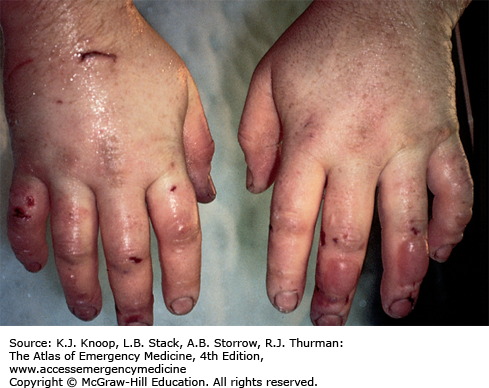
FIGURE 16.7
Thawed Frostbite. Typical appearance of frostbite soon after rewarming. Deep frostbite was caused by wearing mountaineering boots that were too tight in extreme cold at high altitude. Note the deceptively benign appearance of this devastating injury, which ultimately resulted in bilateral below-the-knee amputations. (Photo contributor: Ken Zafren, MD.)
If other injuries are ruled out by history and physical examination, rewarm frostbitten areas in warm water bath (37°C-39°C [98.6°F-102.2°F]). If associated with severe hypothermia, active core rewarming should precede frostbite rewarming. If swelling occurs, surgical consultation is advisable along with compartment pressure measurement to determine the need for fasciotomy. Admit all patients with associated hypothermia or in whom swelling occurs. Superficial frostbite (minimal skin changes and erythema) may be treated by home care with nursing instructions. Deep superficial frostbite (clear, fluid-filled blebs, swelling, pain) may be treated by home care in a reliable patient. Deep frostbite (proximal hemorrhagic blebs, no swelling, no pulses) mandates hospital admission.
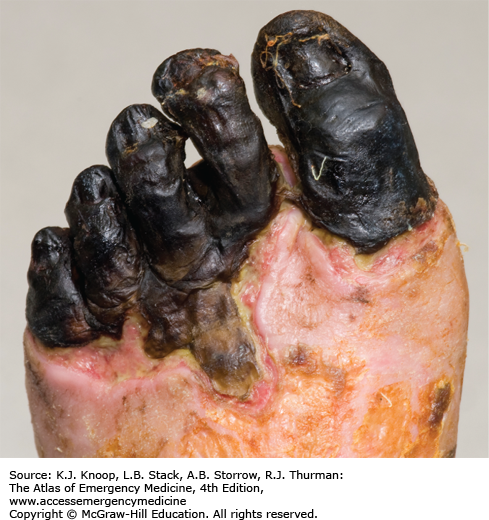
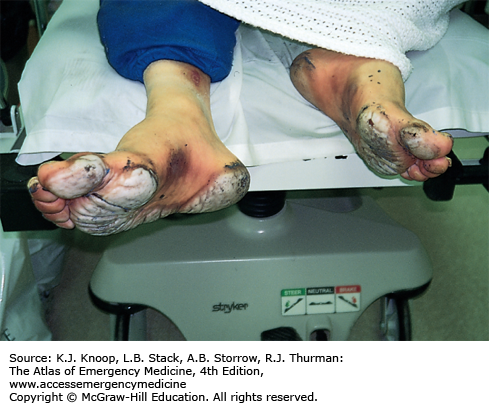
FIGURE 16.8
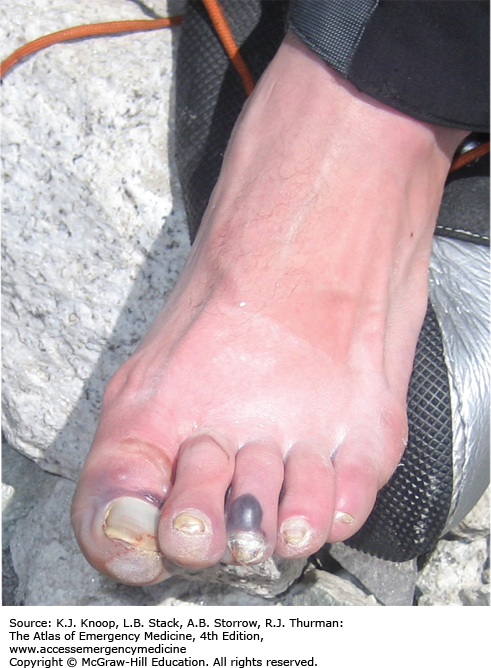
Deep Frostbite. Deep frostbite at Everest Base Camp in Nepal, located at an altitude of 5360 m (17,585 ft), 3 days after exposure at 6400 m (21,000 ft). Dusky appearance and lack of distal blistering on the great toe are poor prognostic signs. The great toe eventually required partial amputation. (Photo contributor: Chris Imray, MD.)
Early transfer of the patient to a center experienced in the care of frostbite injuries (even if far away) should be considered. On the other hand, transfer of the patient to a major medical center that does not generally manage frostbite is seldom in the patient’s best interest.
Treatment of clear versus hemorrhagic blisters is controversial; one approach is to debride clear blisters and use topical aloe vera while leaving hemorrhagic blisters intact.
PERNIO
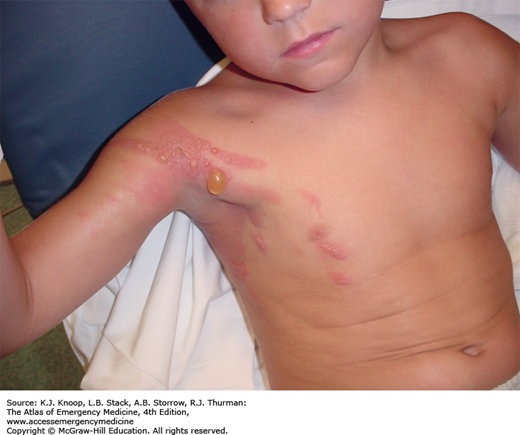
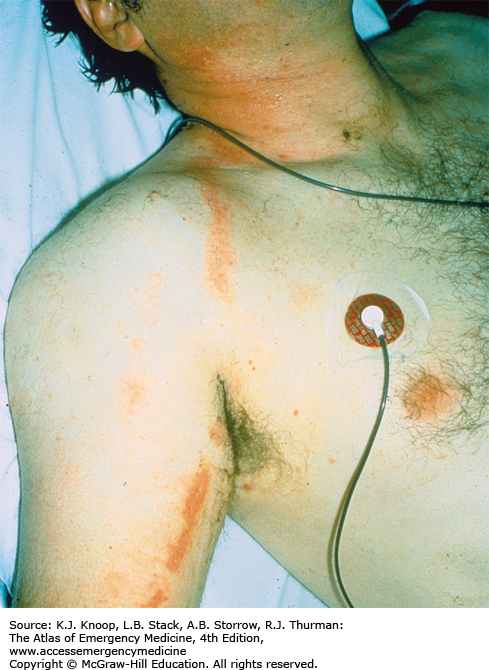
Pernio, also known as perniosis or chilblains, is the result of nonfreezing cold exposure in susceptible individuals. Pernio appears within 24 hours of cold exposure, most frequently on the face, ears, hands, feet, and pretibial areas. A large range of lesions may be seen, with localized edema, erythema, cyanosis, plaques, and blue nodules occasionally progressing to more severe lesions including vesicles, bullae, and ulcerations. The lesions persist for up to 2 weeks and may become chronic. They are typically very pruritic and associated with burning paresthesias. Following rewarming, pernio often takes the form of blue nodules, which are quite tender. In the setting of recent cold exposure, pernio might be confused with the more severe syndrome of trench foot. If the history of cold exposure is not elicited, the differential diagnosis is potentially very broad.
Management is supportive. The skin should be warmed, washed, and dried. Affected extremities can be dressed in soft, dry, sterile dressings and elevated. Nifedipine (20-60 mg daily) may be helpful in chronic cases.
Healing may be followed by hyperpigmentation.
Recurrences are possible following milder cold exposure.
Chilblains may be more frequent in young women, especially in association with Raynaud phenomenon, and may also be associated with underlying dermatologic or vascular disease.
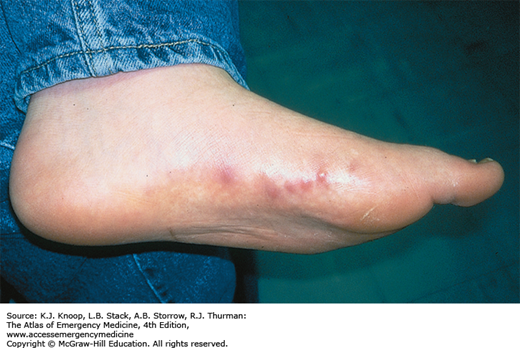
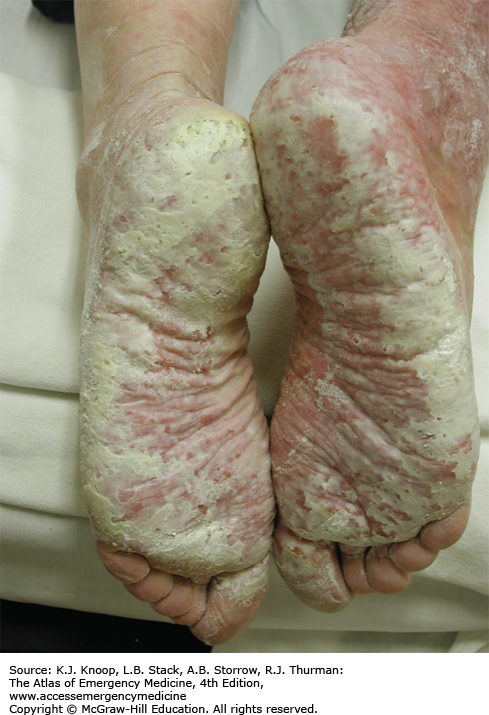
IMMERSION INJURY (TRENCH FOOT)
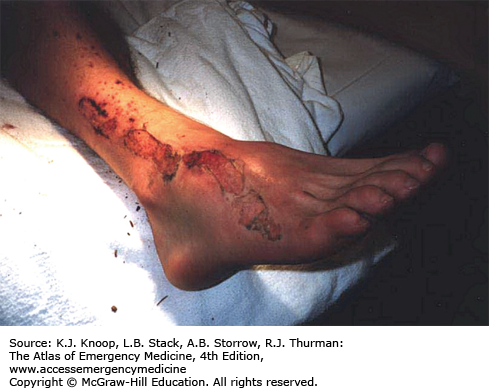
Immersion injury is a peripheral nonfreezing cold injury resulting from exposure to water, usually at temperatures just above freezing. However, the condition can occur during prolonged exposure to any cool wet environment. Dependency and immobility predispose to immersion injury. The degree of injury depends on exposure time and temperature. The first symptoms usually appear within hours; tissue loss may occur after many days of exposure. Prior to rewarming, the distal extremities are numb and swollen. The skin is first red, then changes to pale, mottled, or black. Cramping of the calves may occur. Immersion injury is distinct from tropical immersion foot or warm-water immersion foot as seen in the Vietnam War. Tropical immersion foot was typically seen after 3 to 7 days of exposure to water at 22°C to 32°C. Warm-water immersion foot was seen after 1 to 3 days at 15°C to 32°C. These syndromes were characterized by burning in the feet, pain on walking, pitting edema, and erythema, with wrinkling and hyperhydration of the skin. They resolved completely after rest and removal from the wet environment.
Hypovolemia, hypothermia, and associated injuries are the rule and should be treated first. General treatment of immersion foot (or hand) is similar to that for rewarmed frostbite. Swelling may produce compartment syndrome and require fasciotomy. Most patients require admission to the hospital.
Doppler ultrasound may be useful to identify peripheral pulses, as pulses are often difficult to palpate in affected extremities.
Mixed injuries (frostbite and immersion) are common.
ULTRAVIOLET RADIATION EXPOSURE
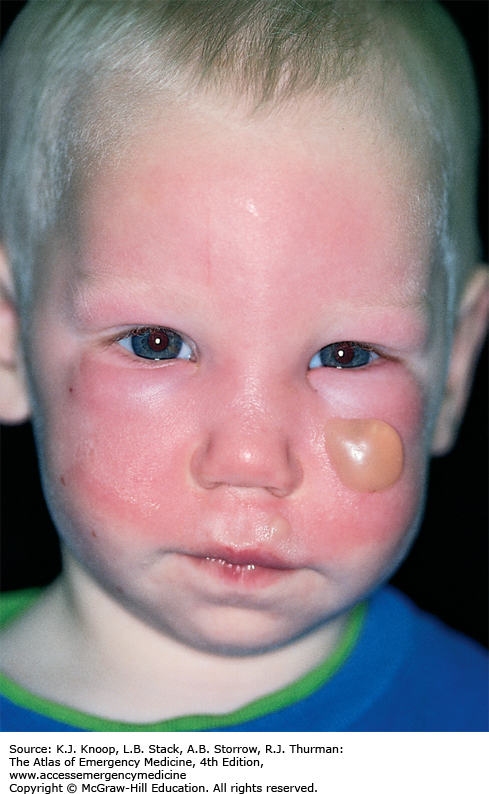
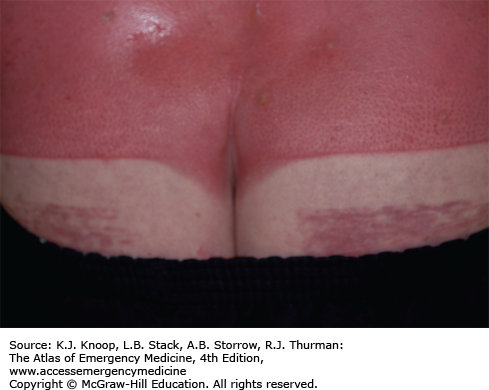
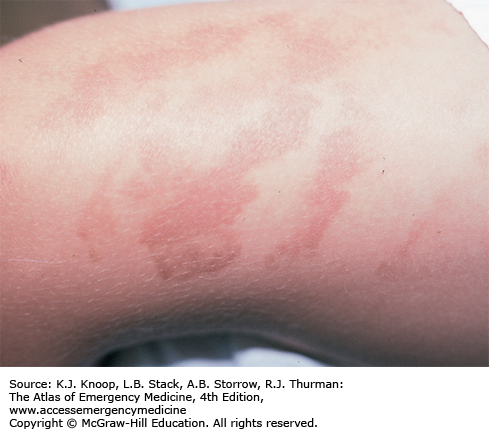
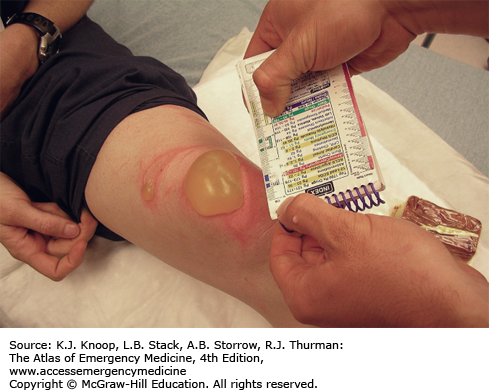
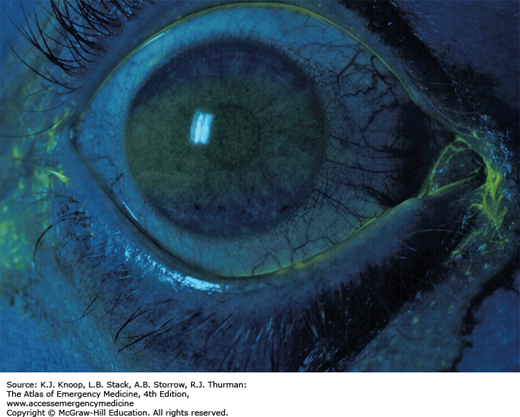
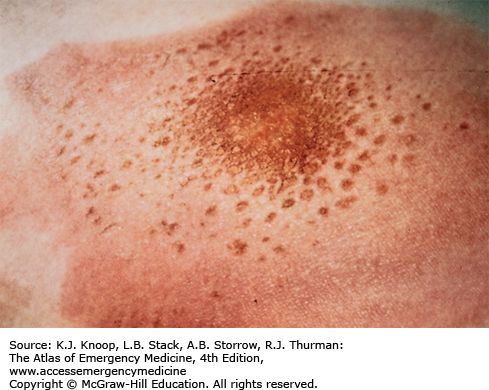
Ultraviolet (UV) radiation causes both acute and chronic skin changes. Sunburn is a partial-thickness burn, which may become a full-thickness injury if infected. “Sun poisoning” is a severe systemic reaction to UV radiation. Patients may complain of nausea, vomiting, headache, fever, chills, and prostration. Excessive UV radiation may cause injury to the cornea and conjunctiva, termed UV keratitis (photokeratitis, snow blindness). This painful condition may occur in skiers, welders, or tanning salon patrons who do not wear proper eye protection.
Photosensitivity reactions (photodermatoses) are of several types. Phototoxic reactions are an abnormal response to UV radiation caused by a substance that is ingested (eg, prescription or over-the-counter medications) or applied to the skin; there is a direct relation between the amount of UV exposure and severity. Photoallergic reactions are clinically similar to contact dermatitis and, like phototoxic reactions, may be precipitated by ingested or applied drugs. Unlike phototoxic reactions, photoallergies may be precipitated by a small amount of light. Phytophotodermatitis is precipitated by skin contact with certain plants followed by exposure to UV radiation.
Phototoxicity should be suspected in any patient with severe or exaggerated sunburn. Photoallergy is easily misdiagnosed as allergic eczema or contact dermatitis, especially since onset is often delayed up to 2 days after exposure. Phytophotodermatitis may mimic severe sunburn or contact dermatitis, especially rhus dermatitis. Endogenous photosensitizers (endogenous photodermatoses) include solar urticaria, porphyria cutanea tarda, polymorphous light eruption, and systemic lupus erythrematosus. These may be provoked by visible light as well as by UV radiation.
Treatment of sunburn and sun poisoning involves standard burn therapy and supportive care. Sunburn is usually a self-limited problem. Cool compresses and nonsteroidal anti-inflammatory drugs may be beneficial. UV keratitis is treated with mydriatic-cycloplegic eye drops to decrease pain; initial examination is made easier with topical anesthetics. Severe cases may require antibiotic ointment, narcotic analgesics, and bilateral eye patches for 12 to 24 hours, though the use of eye patches remains controversial. These patients require 24- to 48-hour follow-up; ophthalmology referral is indicated to rule out retinal damage.
Treatment of photosensitivity reaction has two components: treatment of the sunburn and recognition of the sensitizing agent or endogenous medical condition. Topical steroids and oral analgesics and antipruritics may be helpful. Systemic steroids may be required. Patients with severe reactions should be referred to a dermatologist for possible photo patch testing.
Para-aminobenzoic acid (PABA) in sunscreens may be a photosensitizer and can cause a photoallergic reaction.
The unique properties of individual skin types produce marked differences in response to UV radiation.
Victims of UV keratitis typically present 2 to 12 hours after exposure. As such, patients may present during the night with severe unexplained bilateral eye pain.
FIGURE 16.21
Phytophotodermatitis. This reaction may require aggressive systemic steroid therapy. The case illustrated is a mild one caused by exposure to limes and UVA. A clue to the diagnosis is the patchy distribution with linear edges. More severe reactions resemble rhus dermatitis. (Photo contributor: Lee Kaplan, MD.)
LIGHTNING INJURIES
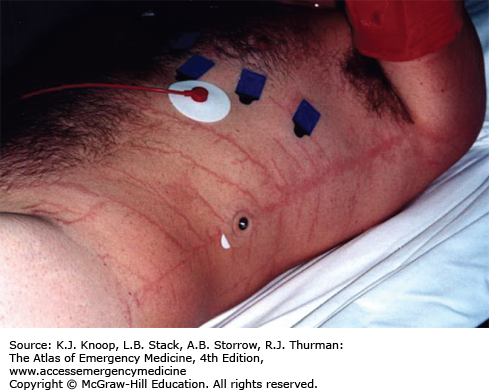
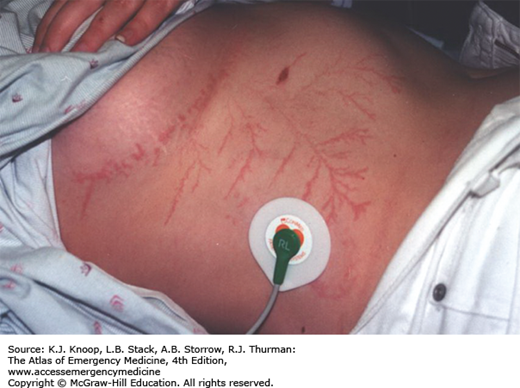
Lightning produces injury from high voltage, heat production, and explosive shock waves. Direct injuries include cardiorespiratory arrest, cardiac arrhythmias, and neurologic abnormalities such as seizures, deafness, confusion, amnesia, blindness, and paralysis. The patient may suffer contusions from the shock wave or from opisthotonic muscle contractions. Chest pain and muscle aches are common. One or both tympanic membranes (TMs) rupture in more than 50% of victims. Cataracts are usually a delayed occurrence. Hematologic abnormalities including disseminated intravascular coagulation (DIC) have been described. Fetal demise may occur.
Burns may result from vaporization of sweat or moist clothing, heating of clothing and metal objects such as climbing equipment, belt buckles or bra wiring, and direct effects of the strike. Linear burns and punctate burns are thermal burns. Feathering burns, also known as ferning or Lichtenberg figures, are not actually burns but may represent superficial bruising. They are pathognomonic of lightning injury.
Diagnosis of lightning injury is straightforward when there is a thunderstorm, when there are witnesses to the strike, or when there are typical physical findings. Lightning on relatively sunny days (without loud thunder) striking a lone victim may produce a confusing picture. The scattering of clothing and belongings may mimic an assault. Side flashes from metal objects and wiring may produce indoor victims during storms.
History and physical examination to rule out associated injuries and standard ED care and workup for critical patients—including cardiac enzymes, urinalysis, and ECG—are necessary. All patients, even those apparently well, require admission for observation since their condition may change over several hours following the lightning strike.
The amount of damage to the exterior of the body does not predict the amount of internal injury.
Since lightning most commonly produces cardiac standstill by means of massive direct current countershock, prompt, spontaneous return of normal heart rhythm (by virtue of cardiac automaticity) is the rule. However, respiratory arrest is often more prolonged. In a triage situation, the normal rules do not apply, since victims breathing spontaneously are already recovering. The rule in lightning strikes is to resuscitate the “dead.” Ventilatory support is often all that is required.
Keraunoparalysis (lightning paralysis) is a condition that is specific to lightning injury and is caused by extreme vasoconstriction, usually in the legs. It resolves spontaneously within a few hours.
Psychological sequelae following lightning injuries are underreported and may include memory loss, difficulty with concentration, and depression.
LARGE TERRESTRIAL ANIMAL ATTACKS
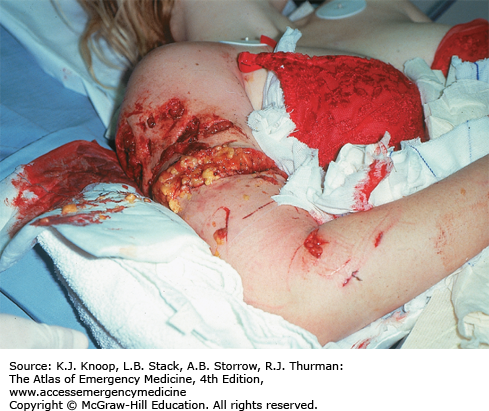
Both domestic and wild animals are known to attack humans. Injuries are caused by combinations of penetrating and blunt trauma. Penetrating injuries can be inflicted by teeth, claws, and horns, while severe blunt injuries may result from the victim being knocked over, trampled or otherwise crushed, being thrown into the air, or dragged. Injuries may involve massive tissue injury or avulsion often associated with neurovascular damage and long bone fractures.
Evaluation and treatment is the same as for any other multiple trauma victim with a high-energy mechanism. After attention to the ABCs, which may be complicated by injuries of the face, neck, or chest, victims should be evaluated for less obvious blunt traumatic injuries. Open fractures and injuries to internal organs may not be clinically apparent initially as with any multitrauma victim.
Wounds often require operative debridement and repair by appropriate specialists. Consider tetanus and rabies prophylaxis along with antibiotic treatment for high-risk wounds.
Bite wounds can penetrate the skull or joint. Displaced teeth or claws may remain imbedded in wounds. Evaluation should include x-rays, ultrasound, and/or CT scanning along with appropriate surgical consultations.
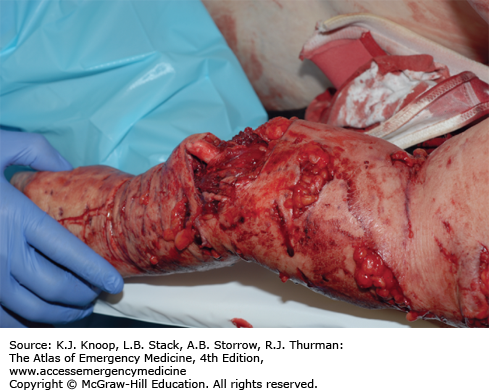
FIGURE 16.31
Cougar Mauling. This patient sustained large wounds as a result of a cougar attack. Here, the weight and force of this large animal has resulted in severe shearing injuries. Penetrating injuries from large animals may be deeper than they appear. (Photo contributor: Alan B. Storrow, MD.)
CORAL SNAKE ENVENOMATION
The most important species of coral snakes (Elapidae family) found in the United States are the eastern coral snake (Micrurus fulvius) and the Texas coral snake (Micrurus tener). Coral snakes have small mouths, and bites are usually limited to fingers, toes, or folds of skin. Due to a less efficient venom apparatus than their Crotalid colleagues, coral snakes generally need to hold on or chew to inflict a significant envenomation. The bite typically produces minimal local inflammation and pain. Paresthesias and muscle fasciculations are common. Systemic symptoms resulting from the powerful neurotoxic effects of the venom can include tremors, drowsiness, euphoria, marked salivation, and respiratory distress. Cranial nerve involvement, manifested by slurred speech and diplopia, may be followed by bulbar paralysis with dysphagia and dyspnea. Death may result from respiratory and cardiac arrest. Onset of severe symptoms may be delayed up to 12 hours, but may also be rapidly progressive.
In contrast to pit viper envenomation, prehospital application of a constrictive bandage may be of benefit in limiting the spread of neurotoxic coral snake venom. Severe systemic symptoms following envenomation by Elapidae may be delayed and cannot be accurately predicted by local wound reactions. It is therefore recommended that four to six vials of antivenom be administered for all suspected envenomations by eastern or Texas coral snakes. Treatment of western coral snake (Micruroides euryxanthus) bites is purely supportive. Tetanus prophylaxis should be addressed.
Treatment with antivenom should be initiated early in cases of eastern coral snake bites, since symptoms are often delayed and severe.
The adage “red on yellow, kill a fellow; red on black, venom lack” applies to all coral snakes found in the United States but does not hold true in other parts of the world.
As many as 60% of North American coral snake bites do not result in envenomation of the victim.
PIT VIPER ENVENOMATION
The pit vipers (Crotalidae family) indigenous to the United States include rattlesnake species, cottonmouths, and copperheads. Physical characteristics of pit vipers include a triangular head, heat-sensing pits, elliptical pupils, and a single row of subcaudal ventral scales. Pit viper venom is complex and produces hematologic, cardiovascular, and neuromuscular effects. Clinically, pit viper envenomations are divided into four categories. Snakebites without envenomation are characterized only by the direct tissue damage caused by the strike. Minimal envenomations consist of fang marks and local swelling but no systemic symptoms. Moderate envenomations include progression of tissue changes beyond the immediate location of the bite and/or systemic symptoms and mild changes in coagulation parameters. Severe envenomations include marked local and progressive swelling and significant systemic symptoms and coagulopathy (eg, subcutaneous ecchymosis and/or signs of bleeding).
Prehospital management of pit viper bites should include immobilization and rapid transport without delay. Lymphatic constriction bands and extractor devices should not be used. These devices may worsen focal complications of envenomation. Tourniquets and local incision are ineffective and generally do more harm than good. Electric shock, cryotherapy, and mouth suction are not recommended. ED management includes resuscitation, establishing a physiologic baseline, and determining the need for antivenom. CroFab (Savage Labs), a recombinant antivenom effective in neutralizing venom toxins, is the treatment of choice. Indications for antivenom administration include any progression of swelling, erythema, or ecchymosis beyond the immediate bite area, presence of any systemic signs of envenomation, or any coagulation abnormalities and/or bleeding complications associated with envenomation. The dose of antivenom increases with the severity of envenomation. The package insert details the current recommendations for antivenom administration and allergy testing, but in general treatment is initiated with 4 to 6 vials of CroFab intravenously with further dosing regimen based on clinical course. Compartment syndrome is a possible complication of envenomation, and should initially be treated with antivenom. Fasciotomy should be performed only if compartment pressures are greater than 30 mm Hg in spite of antivenom treatment. Patients who do not develop evidence of envenomation after 8 hours of observation may be safely discharged home with close follow-up. Tetanus prophylaxis should be addressed and given when necessary.
Up to half of all pit viper bites may be “dry” (without any injection of venom).
Intramuscular epinephrine (0.3 mg 1:1000) given prior to administration of horse serum antivenin may reduce the potential allergic response.
FIGURE 16.39
Mojave Green Rattlesnake. The Mojave green is found in the southwestern United States and Mexico. There are two subspecies, Types A and B, with Type A thought to have the most toxic venom of all North American snakes. Unlike most rattlesnake venoms, Mojave Type A venom contains a potent neurotoxin. (Photo contributor: Mike Cardwell, MS.)
FIGURE 16.40
Red Diamond Rattlesnake. The elliptical pupils and heat-sensing pits in this red diamond rattlesnake are characteristic of pit vipers. (Photo contributor: Sean P. Bush, MD.)