86 Emergent Valvular Disorders
In the critical care setting, there are two distinct presentations of valvular heart disease: acute valve dysfunction resulting in acute heart failure and chronic valve disease with decompensation due to increased metabolic demands (Table 86-1).1 Valve regurgitation is the most common type of acute valve dysfunction. Valve stenosis, with rare exceptions, is a chronic slowly progressive disease. However, in patients with asymptomatic chronic valve stenosis, acute deterioration can occur if there is a superimposed hemodynamic burden. For example, patients with previously asymptomatic mitral stenosis may present with pulmonary edema in the setting of a systemic infection. Another example is the elderly adult with asymptomatic aortic stenosis who presents with cardiogenic shock in the setting of an acute gastrointestinal bleed. Prosthetic valve dysfunction also can present emergently, particularly mechanical valve thrombosis.
TABLE 86-1 Causes of Acute Valve Dysfunction
| Mitral regurgitation | Myxomatous disease with flail leaflet |
| Spontaneous chordal rupture | |
| Endocarditis | |
| Acute myocardial infarction: Papillary muscle rupture Regional wall motion abnormality LV dilation and systolic dysfunction | |
| Aortic regurgitation | Endocarditis |
| Spontaneous rupture of a congenital fenestration | |
| Aortic dissection | |
| Tricuspid regurgitation | Endocarditis |
| Penetrating chest trauma | |
| Blunt chest wall trauma | |
| Prosthetic valves | Endocarditis |
| Valve thrombosis | |
| Paravalvular dehiscence | |
| Leaflet tear |
 Mitral Regurgitation
Mitral Regurgitation
Etiology
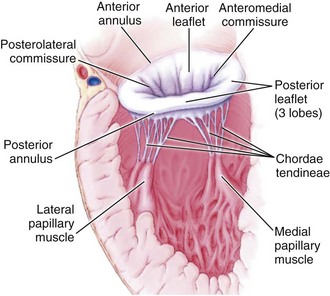
Mitral regurgitation may be caused by disease or distortion of any component of the mitral valve apparatus—the mitral annulus, leaflets, chordae, and papillary muscles—as well as by alterations in left ventricular (LV) geometry or systolic function (Figure 86-1).2 Primary causes of chronic mitral regurgitation include myxomatous valve leaflets (mitral valve prolapse) and rheumatic disease. Chronic secondary mitral regurgitation may be due to dilated cardiomyopathy or to coronary artery disease with regional or global LV dysfunction.
Acute mitral regurgitation also may be due to involvement of the valve leaflets or the left ventricle. Patients with myxomatous mitral valve disease may develop acute regurgitation due to spontaneous chordal rupture.3 Bacterial endocarditis results in acute mitral regurgitation due to destruction of valve tissue, often with leaflet perforation. Moderate to severe mitral regurgitation due to papillary muscle involvement or regional myocardial dysfunction complicates 12% of acute myocardial infarctions and is associated with an increased risk of heart failure or death.4
Clinical Presentation
Although patients with chronic mitral regurgitation may be asymptomatic for many years, the regurgitant lesions impose a volume load on the left ventricle, because an increased total stroke volume is needed to maintain a normal forward cardiac output. Left ventricular volume overload results in progressive LV dilation and may lead to an irreversible decline in ventricular contractility, even in the absence of clinical symptoms. Evaluation of ventricular contractility is problematic in patients with mitral regurgitation, given that measures of ventricular performance are affected by preload and afterload.5 However, based on outcomes after mitral valve surgery, the empirical parameters of ventricular end-systolic dimension and ejection fraction can be used to optimize the timing of surgical intervention. Thus, patients with moderate to severe chronic regurgitation undergo periodic echocardiography, with valve repair or replacement recommended when the end-systolic dimension is ≥ 40 mm and the ejection fraction is ≤ 60%.6
Chronic mitral regurgitation usually is well tolerated even when there is a superimposed hemodynamic load such as systemic infection, pregnancy, or trauma. However, mitral regurgitant severity may acutely worsen by at least two mechanisms. An increase in afterload, for example with a hypertensive crisis, may increase regurgitant severity due to an increased driving pressure from the left ventricle to the left atrium. Alteration in LV geometry, for example with ventricular dilation due to decompensated heart failure, may change the orientation of the papillary muscles such that leaflet closure is impaired, resulting in a larger regurgitant orifice area.7 In this situation, a vicious cycle may ensue where LV dilation worsens mitral regurgitant severity, which increases LV dilation, and so forth.
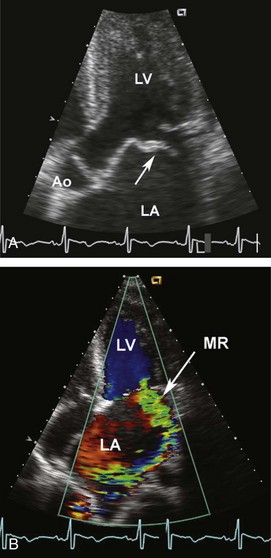
Acute mitral regurgitation presents with acute pulmonary edema and is a surgical emergency (Figures 86-2 and 86-3). Mitral chordal rupture results in the acute presentation of heart failure, often in patients who were unaware of a diagnosis of mitral valve prolapse. Patients with mitral valve perforation due to endocarditis present with pulmonary edema superimposed on signs and symptoms of endocarditis. Papillary muscle rupture or dysfunction after MI usually presents several days after acute MI; in some cases, the initial presentation is acute pulmonary edema, with the MI being clinically silent.
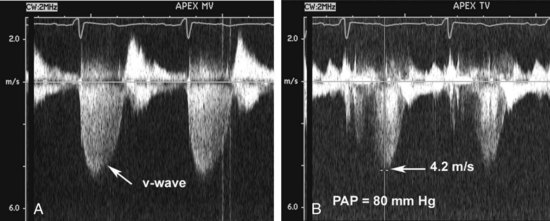
Figure 86-3 In the same patient as Figure 86-2, severe mitral regurgitation was recorded with continuous wave Doppler ultrasound (A). The rapid rise in left atrial pressure due to the regurgitant jet entering the left atrium results in a rapid decline in Doppler velocity in late systole—the Doppler equivalent of the v-wave seen on a pulmonary wedge pressure tracing. The continuous-wave Doppler recording of maximum tricuspid regurgitant jet velocity (B) of 4.2 m/sec indicates a right ventricular–to–right atrial pressure difference of 70 mm Hg. The patient’s right atrial pressure was estimated to be 10 mm Hg, based on size and respiratory variation in the inferior vena cava, so the estimated pulmonary systolic pressure is 80 mm Hg.
Diagnosis
A high level of clinical suspicion is needed to make the diagnosis of acute mitral regurgitation (Table 86-2). Acute pulmonary edema often obscures the signs and symptoms of the underlying disease process. The classical finding is a holosystolic murmur at the apex, radiating to the axilla. Although there is some correlation between the loudness of the murmur and regurgitant severity with chronic regurgitation, the murmur may be soft with acute severe mitral regurgitation. In patients with severe mitral regurgitation after MI, a murmur cannot be appreciated at all in up to 50% of patients.
TABLE 86-2 Diagnostic Approach to Acute Valve Dysfunction
| Physical examination | Unreliable |
| Consider valve dysfunction in all patients with pulmonary edema | |
| Echocardiography (transthoracic) | Accurate diagnosis of etiology of disease |
| Quantitation of severity of stenosis or regurgitation | |
| Measurement of ventricular ejection fraction | |
| Estimation of pulmonary pressures | |
| Transesophageal echocardiography | Sensitive for detection of valvular vegetations |
| Detection of paravalvular abscess | |
| Essential for prosthetic mitral valve dysfunction | |
| Useful for prosthetic aortic valve dysfunction | |
| Right heart catheterization | Not reliable for diagnosis of valve disease |
| May be helpful for optimizing loading conditions | |
| Chest computed tomography | Sensitive and specific for diagnosis of aortic dissection |
| Angiography | Used when coronary angiography is needed |
Management
In patients with chronic mitral regurgitation and heart failure, management is directed at treating the process leading to decompensation and optimizing loading conditions (Table 86-3). For example, in a patient with a systemic infection, treatment of the infection, control of fever and tachycardia, and invasive monitoring to optimize preload and afterload are utilized. Medical therapy typically includes afterload reduction with nitroprusside or other vasodilators and preload reduction with diuretics.8,9 The goal is to support the patient through the period of decompensation. Typically, hemodynamics return to the baseline compensated state after the acute illness.
TABLE 86-3 Therapeutic Approach to Acute Valve Dysfunction
In contrast, acute severe mitral regurgitation is a surgical emergency. Mortality is extremely high without restoration of valve competence; even with prompt valve surgery, 30-day mortality is 23%.10 Medical stabilization should occur concurrently with consultation by a cardiac surgeon. Acutely, placement of an intraaortic balloon pump (IABP) provides optimal afterload reduction while improving diastolic coronary blood flow.
The timing of surgery for endocarditis depends on the disease course in that individual, but most centers now advocate early surgical intervention in the patient with heart failure or severe valve regurgitation to prevent progressive valve damage and paravalvular abscess formation.11,12 In a large prospective multicenter study, early surgery was associated with a lower mortality than medical therapy (12% versus 21%).13 Valve repair is preferred but may not be possible, depending on the extent of tissue destruction. Early surgery is particularly beneficial in patients with paravalvular complications or systemic embolization.14
In patients with acute ischemic mitral regurgitation, treatment depends on the exact etiology of valve dysfunction.15 In patients with acute mitral regurgitation due to a regional wall-motion abnormality, myocardial function may improve after percutaneous revascularization.16 In these patients, use of an IABP and medical therapy may be advantageous during the acute episode, with weaning of therapy as myocardial function improves.
Mitral regurgitation due to partial or complete papillary muscle rupture requires surgical intervention. Although the risk of surgery is high, with an operative mortality rate of about 50%, outcome is even worse with medical therapy, with a mortality of 75% at 24 hours and 95% within 2 weeks after complete papillary muscle rupture.17 With the use of echocardiography, partial papillary muscle rupture can be recognized; prognosis in these patients depends on the extent of myocardial damage and severity of mitral regurgitation.18 With partial papillary muscle rupture, some surgeons prefer to stabilize the patient and delay surgery for 6 to 8 weeks after MI to avoid operating on the necrotic myocardial tissue. However, many patients cannot be stabilized, so acute intervention must be considered. Again, valve repair is preferred, but myocardial necrosis may necessitate valve replacement. Risk factors for surgery include older age, female gender, and poor LV systolic function. In some patients, the risk of surgical intervention may be so high as to be futile.
 Aortic Regurgitation
Aortic Regurgitation
Etiology
Chronic aortic regurgitation most often is due to a congenital bicuspid valve, rheumatic valve disease, or aortic root dilation. There are numerous causes of aortic root dilation, including hypertension, cystic medial necrosis, Marfan syndrome, and a bicuspid aortic valve.19 The most common causes of acute aortic regurgitation are endocarditis, rupture of a congenital fenestration, and acute aortic dissection.1 Endocarditis results in aortic regurgitation by destruction of the valve leaflet tissue, with a high percentage of cases also having paravalvular abscess formation. Aortic dissection results in acute aortic regurgitation either due to enlargement of the aortic annulus or to extension of the dissection into the valve region, resulting in a flail aortic valve leaflet.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree