122 Emergency Delivery and Peripartum Emergencies
• If shoulder dystocia is encountered, attempt the McRoberts maneuver with suprapubic pressure while simultaneously calling for obstetric assistance.
• Practitioners underestimate blood loss in postpartum hemorrhage by as much as 50%.
• Uterine inversion is relatively uncommon but is associated with significant morbidity if not recognized and treated promptly.
• Up to 87% of patients with uterine rupture have no pain, and up to 89% have no vaginal bleeding.
• Perimortem cesarean delivery is indicated in gravid patients if more than 24 weeks pregnant and if arrest continues after 4 to 5 minutes of cardiopulmonary resuscitation.
• The clinical examination of a term pregnant patient is notoriously unreliable.
• Treatment of anaphylactoid syndrome of pregnancy is similar to that for sepsis and disseminated intravascular coagulation and should begin immediately; the diagnosis is one of exclusion, and patients must be treated promptly to survive.
Emergency Delivery
Pathophysiology
For a description of potential malpresentation, along with management recommendations, see www.expertconsult.com
Presenting Signs and Symptoms
In the emergency department (ED), the majority of patients seen in labor are in the latter part of stage I or stage II.1 Precipitous delivery is a common occurrence because most patients whose labor progresses more slowly will have time to get to their designated obstetric facility.
Treatment
For more information on the management of difficult labor, see www.expertconsult.com
If delivery is imminent, the patient will have to remain in the ED. A gynecologic bed with lithotomy position capability is ideal, and a resuscitation bay with greater accessibility and equipment is recommended. A radiant warmer and appropriate airway equipment should be available. Positioning of the mother may require an approximate 10-degree tilt to the left to prevent uterine pressure on the inferior vena cava and associated hypotension. When crowning occurs, the mother should be instructed to push along with the contractions, with the physician positioned in front of the introitus ready to accept the fetus. As soon as the head is accessible, continuous gentle countertraction should be administered to maintain it in a flexed position. This technique provides control of an explosive delivery, as well as avoidance of the high morbidity associated with fetal neck hyperextension. Though once recommended, the modified Ritgen maneuver has recently been shown to be associated with an increased rate of third-degree lacerations and episiotomy in comparison with a “hands-off” approach.2 Similar rates of perineal tears were found for each modality.
Postpartum Hemorrhage
Epidemiology
Hemorrhage is a significant cause of maternal morbidity and is the second most common cause of peripartum deaths (following amniotic fluid embolism). Hemorrhage was a direct cause of more than 18% of 3201 pregnancy-related maternal deaths in the United States from 1991 to 1997.3 Worldwide, hemorrhage has been identified as the single most important cause of maternal death and is responsible for almost half of all postpartum deaths in developing countries.4
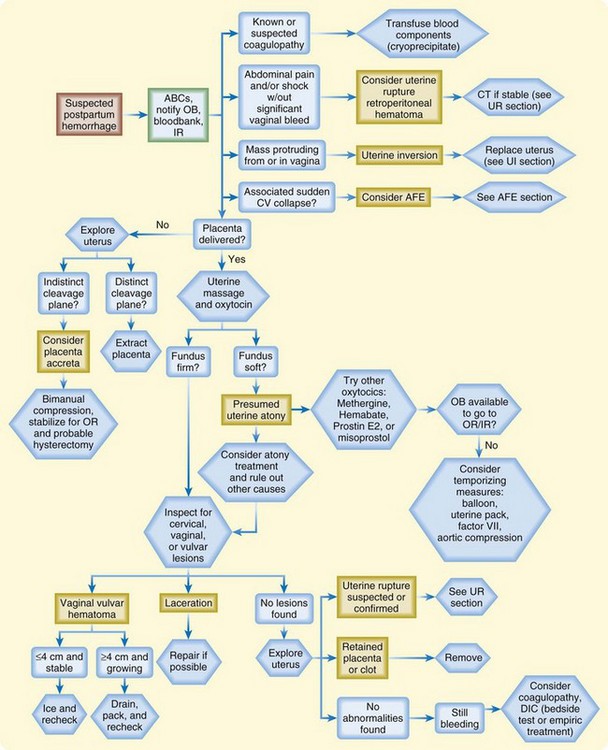
Postpartum hemorrhage is the term used to describe excessive blood loss after delivery. Classically, it is defined as more than 500 mL of blood loss in a vaginal delivery or more than 1000 mL of blood loss in a cesarean delivery; however, careful quantitative measures reveal that blood loss in the range of 500 to 1000 mL is actually average for both types of delivery. Of note, practitioners often underestimate blood loss by as much as 50%.5 For the ED physician, the most important causes of hemorrhage in the postpartum period are birth trauma, uterine atony, uterine rupture, and uterine inversion.
Presenting Signs and Symptoms
In a reported case series on uterine inversion, the most common signs were shock and hemorrhage.6 With a complete inversion, the prolapsed uterus may be visible as a large, dark red polypoid mass within the vagina or protruding through the introitus. If the fundus remains within the vagina, the diagnosis may be suspected because of dimpling, indentation, or absence of the uterine fundus on abdominal examination or because a mass is palpated in the cervix on bimanual examination. Establishing the diagnosis of incomplete inversion can be quite difficult; severe hypotension, postpartum hemorrhage, and subtle abnormalities on abdominal examination may be the only clues.
Uterine rupture is also a difficult clinical diagnosis and should be considered in any patient with unexplained peripartum hemorrhage or hypotension. The classic findings of uterine rupture are “ripping” or “tearing,” suprapubic pain and tenderness, absence of fetal heart sounds, recession of the presenting parts, and vaginal hemorrhage. Signs and symptoms of hypovolemic shock and hemoperitoneum may follow. This classic manifestation is actually rare; 87% of patients with uterine rupture have no pain and 89% have no vaginal bleeding. Pain is also an unreliable finding because of the altered response to noxious intraperitoneal stimuli by a stretched abdominal wall. Fetal distress is the most consistent finding (80% to 100%), with fetal bradycardia being the most common sign.7 Most reports of uterine rupture describe patients with normal blood pressure or even elevated blood pressure without tachycardia. Abnormal maternal vital signs are late indicators of severe hemorrhage. The most important risk factor for uterine rupture is a previous uterine scar; other factors are listed in Box 122.1.
Differential Diagnosis and Medical Decision Making
Without palpation or visualization of a frankly prolapsed uterus, it may be difficult to differentiate uterine inversion from severe atony. Heavy bleeding may make visualization of the cervix impractical. In addition, accurate abdominal palpation for a uterine fundus may be impossible in an obese patient. Depending on factors such as patient stability, resources, and diagnostic uncertainty, ultrasonography or laparotomy may be necessary. In stable patients in whom the diagnosis is uncertain and resources are available, prompt ultrasound scanning may be helpful.9 Ultrasonography may be able to identify retained products or clot in the uterus, but manual exploration is still needed. Ultrasound can also help detect peritoneal free fluid suggestive of uterine rupture. In selected circumstances in stable patients, a computed tomography scan can be useful in making the diagnosis in those with postpartum hemorrhage (retroperitoneal hematoma). If the accompanying hemorrhage or shock is sufficiently alarming to require immediate exploration, the correct diagnosis may be established only at laparotomy.
Treatment
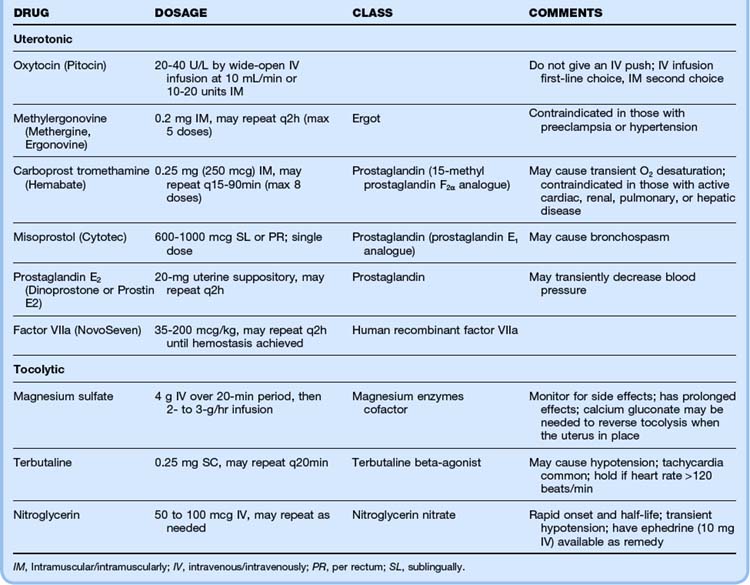
The most important aspects of managing postpartum hemorrhage are obtaining hemostasis and treating shock, including supplemental oxygen, placement of two large-bore intravenous (IV) lines, hemodynamic monitoring, and volume replacement. In addition, blood should be typed and crossmatched and 4 to 6 units of packed red blood cells should be available. Consultation with the obstetrics service should be arranged. Along with the initial resuscitation, bimanual massage and IV oxytocin should be initiated (Fig. 122.1 and Table 122.1). A Foley catheter should be placed.
First-line interventions for atony are part of the initial management of postpartum hemorrhage—namely, initiation of bimanual uterine compression, IV oxytocin, and clearing of products of conception and clots from the uterus. If bleeding persists after the initial interventions, additional uterotonic medications should be given (see Table 122.1). The choice of agent may be influenced by the side effect profile, but the best drug is probably the agent that is the most quickly available in the ED. Interventional radiology may be beneficial because embolization may control the bleeding. In any case, temporizing measures may be required until definitive intervention (Table 122.2).
Table 122.2 Temporizing Measures for Hemostasis of Postpartum Hemorrhage
| METHOD | PROCEDURE | COMMENTS |
|---|---|---|
| Uterine packing | Layer sterile gauze within the uterus, with the distal end going out through the os | May adhere to the uterine wall and removal required; does not allow monitoring of ongoing bleeding; start prophylactic antibiotics |
| Balloon tamponade | If available and time allows, use bedside ultrasonography to confirm that the balloon is beyond the internal os before inflation to avoid damage to the cervical canal; give prophylactic antibiotics and continue oxytocin infusion | |
| Foley catheter | Insert a large bulb catheter (24 French) into the uterus Instill with 80-100 mL of saline Pack the vagina to avoid expulsion of the catheter | Multiple catheters may be needed (in a sterile overbag), which makes the inner lumen difficult to monitor |
| SOS Bakri balloon | Insert into the uterus Instill 300-500 mL of saline through the stopcock Pack the vagina | Best option if available; allows direct measurement of ongoing bleeding via the open inner lumen; developed for postpartum hemorrhage; balloon conforms to the shape of the uterine cavity |
| Sengstaken-Blakemore tube | Cut off the distal (“stomach”) end of the tube Insert inside the uterine cavity Infuse 75-300 mL of saline Pack the vagina to avoid expulsion of the tube | Does not conform to the shape of the uterine cavity; with the end cut off, proximal bleeding can be monitored through the lumen; may be available from the gastrointestinal department laboratory if not available in the emergency department |
| Rusch catheter | Using a 60-mL bladder syringe, inflate the balloon via the drainage port with 150-500 mL of saline Pack the vagina to avoid expulsion of the tube | Urologic catheter used for bladder stretching; may be available in the urology department |
| Condom catheter | Slide the condom over the end of the Foley catheter and tie it off with string to close the end Inflate with 250-500 mL of saline and clamp the end Pack the vagina to avoid expulsion of the tube | A sterile rubber catheter is fitted with a condom |
| Vaginal packing | Pack the vagina with a blood pressure cuff placed inside a sterile glove Increase pressure to 10 mm Hg above systolic blood pressure | Various techniques have been described; concern for bleeding proximal to the vaginal pack |
| Noninflatable antishock garment | Begin application at the ankles and progress sequentially up to the abdomen | Adjust the panels if any discomfort or dyspnea; contraindicated in women with heart failure or mitral stenosis |
Uterine tamponade with sterile gauze and balloon tamponade are commonly used temporizing measures. Uterine balloon tamponade has been described with large Foley catheters, Sengstaken-Blakemore tubes, condom catheters, sterile gloves, and Rusch urologic catheters, as well as with catheters specifically designed to be used for uterine tamponade in patients with postpartum hemorrhage (SOS Bakri tamponade balloon).10
Uterine Inversion
Management of uterine inversion has two important components: treatment of hemorrhagic shock and immediate repositioning of the uterus (Fig. 122.2). Resuscitation should be initiated immediately and continued while attempts are made to reposition the uterus manually. If oxytocin is being infused, it should be stopped once uterine inversion is suspected.
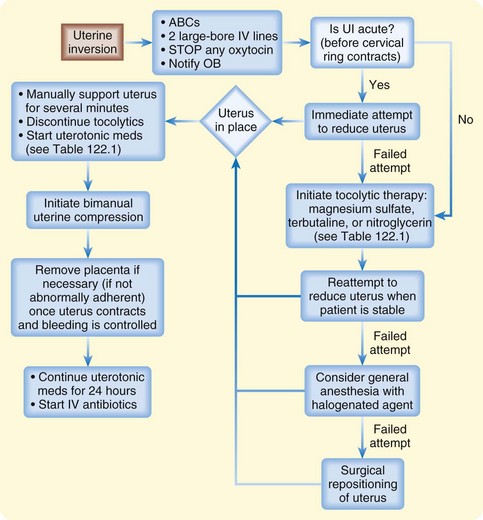
Fig. 122.2 Algorithm for uterine inversion management strategies.
ABCs, Airway, breathing, and circulation; IV, intravenous; OB, obstetrics; UI, uterine inversion.
The success of nonsurgical replacement depends on completion before the myometrium regains its tone. The reported rate of successful immediate reduction is between 40% and 80%.11 If initial measures are delayed or fail to relieve the condition, the inversion may progress to the point at which operative treatment or even hysterectomy is necessary.
The most common nonsurgical replacement method is a variation of the Johnson maneuver.12 The prolapsed uterus is cupped in the operator’s palm, and firm upward pressure is applied to move the uterus up through the cervix along the natural curve of the pelvis toward the umbilicus until it is in place. Usually when the inverted mass is pushed upward, the uterus automatically reverts, with the fundus returning to its anatomic position. If the placenta has not separated, it should not be removed.
If initial repositioning is unsuccessful, myometrial relaxation with pharmacologic agents should be attempted. Magnesium sulfate, terbutaline, and nitroglycerin (attractive because of its easy availability and short half-life) are the agents most commonly used (see Table 122.1).13 Attempts at manual repositioning of the uterus should continue.
Uterine Rupture
For unstable patients, prompt, aggressive resuscitation is an important temporizing measure until definitive surgical repair is performed. Fetal morbidity almost invariably occurs because of catastrophic hemorrhage, fetal anoxia, or both. In a study of 99 cases of uterine rupture, the best fetal outcomes were noted when surgical delivery was accomplished within 17 minutes from the onset of fetal distress.7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree