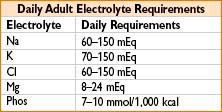
(LR, NS, & hetastarch → shown to ↑ neutrophil activation; blood, 7.5% hypertonic saline, & albumin did not have this effect)
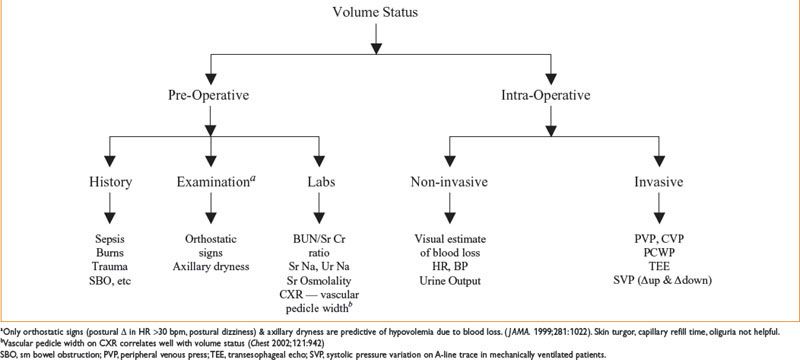
Figure 9-1. Assessment of volume status.

Colloid osmotic pressure (COP) = net osmotic pressure exerted by plasma proteins

Notes on Specific Fluids
• 5% dextrose—used to replace free water; isotonic to plasma but rapidly becomes free water (dextrose is metabolized); used to treat dehydration losses; limited intraoperative use
• Lactated Ringer (LR)—most widely used solution; lactate metabolized in liver to CO2 & water; unsuitable for pts with end-stage liver dz; mixing LR with PRBCs
→ leads to clotting (2° to LR’s calcium content).
→ Interestingly, LR may be superior to NS in patients undergoing renal transplant due to lower incidence of acidosis and hyperkalemia (Anesth Analg. 2005;100:1518–1524)
• 0.9% saline or “normal” saline (NS)—widely used in OR; useful in neurosurgery because of its osmolality; widely used in pts with renal failure; administration of large volumes of NS leads to hyperchloremic acidosis
• Hypertonic Saline (3%, 7.5%, and 23.4%)—two well-defined uses: (1) Intravascular volume expansion in hypovolemic shock although a recent trial did not show evidence of benefit with 7.5% HS (Ann Surg. 2011;253:231–241), (2) reduce cerebral blood volume & ICP. While high quality data is not available, current evidence suggests that HS is more effective than mannitol in reducing ICO in traumatic brain injury (TBI) (Crit Care Med. 2011;39:554–559)
• Albumin—5% & 25% conc. available; circulatory half-life normally 16 hrs (as short as 2–3 hrs in pathophysiologic conditions); made from pooled human blood; minimal/no risk of transmitting infections; 5% albumin = isotonic with plasma; concerns that albumin ↑ mortality are unfounded (SAFE study. N Engl J Med. 2004;350:2247). However, subgroup analysis of the SAFE study suggests that albumin may be associated with worse outcomes in patients with head injury. A follow-up of the head injury patients in the SAFE study suggested that outcomes at 24 mos remained inferior in the albumin group (N Engl J Med. 2007;357:874–884). Although the physiologic rationale is unclear, albumin should probably be avoided in patients with severe TBI. There is some data that supports the combination of 25% albumin boluses and furosemide infusions in hypoproteinemic patients with acute lung injury in an attempt to increase COP and improve oxygenation (Crit Care Med. 2005;33:1681–1687)
• Hydroxyethyl starch (hetastarch)—high-molecular-weight synthetic colloid, ↑ plasma COP up to 2 d; available as 6% solution in NS or LR; renally excreted; side effects → elevates serum amylase, anaphylactoid rxns, coagulopathy (inhibits platelets, decreases factor VIII and vWF); to minimize plt inhibition → restrict dose to 20 mL/kg/d. Should be avoided in patients with sepsis as they may be associated with a significantly increased risk of renal dysfunction in this setting (N Engl J Med. 2008;358:125–139)
• Dextran—dextran 40 & dextran 70 (numbers refer to average molecular mass of solution); side effects include anaphylactoid rxn, ↑ bleeding time, interference with blood crossmatching; rare cases of noncardiogenic pulmonary edema; renal obstruction/acute renal failure.
Dextran 40 → used in vascular surgery to prevent thrombosis;
Dextran 70 → used for same indications as 5% albumin
• Voluven (HES 130/0.5)—Voluven is a relatively new hetastarch with a low molecular weight and a low degree of substitution that theoretically may be associated with a smaller bleeding risk compared to older starches. However, this may not be true since some of the studies reporting a lower bleeding risk have been retracted due to scientific misconduct, and a recent in vitro study did not find a significantly lower bleeding risk (Intensive Care Med. 2011;37:1725–1737). In light of the recent multi center Scandinavian trial of tetrastarch in patients with sepsis or septic shock (the 6S trial) and the previous VISEP trial, the use of hydroxyethyl starch solutions should be considered contraindicated in patients with sepsis. (N Engl J Med 2012. DOI: 10.1056/NEJMoa1204242). It is unlikely therefore, that Voluven represents a major advance in resuscitation.
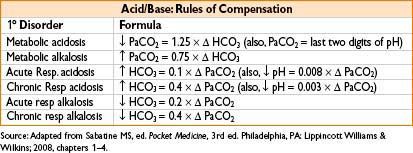
APPROACH TO ACID–BASE ANALYSIS
Check arterial pH
pH <7.40 (acidic)
→ PCO2 >40 respiratory acidosis
→ Hypoventilation (e.g., overdose)
→ Obstruction (COPD)
→ Decr respiratory drive (EtOH, drugs)
→ Neuromuscular disease
→ PCO2 <40 metabolic acidosis
→ Check gap: Na – (bicarb + Cl)
→ Normal gap (12 ± 2)
→ Decreased bicarb
→ Diarrhea
→ Renal tubular acidosis
→ Increased gap (>12)
→ Methanol
→ Uremia
→ DKA (diabetic ketoacidosis)
→ Paraldehyde
→ INH (isoniazid)
→ Lactic acidosis
→ Ethylene glycol (antifreeze)
→ Salicylates
pH > 7.40 (alkalemic)
→ PCO2 <40 respiratory alkalosis
→ Hyperventilation
→ PCO2 >40 metabolic alkalosis
→ Vomiting
→ Diuretics
→ Antacid abuse
→ Increased aldosterone

ELECTROLYTES
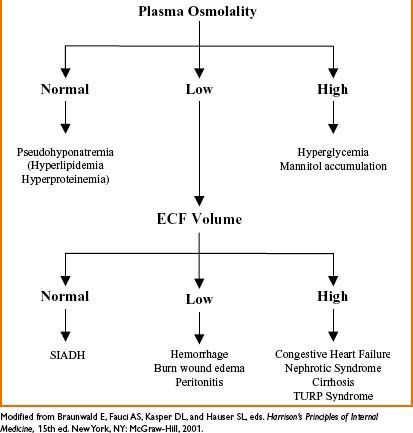
Hyponatremia (Na+ <135 mEq/L)
• Etiology (see Fig. 9-2 below)
Figure 9-2. Etiology of hyponatremia.
• Symptoms—nausea, vomiting, weakness, muscle cramps, visual disturbances
↓ level of consciousness, agitation, seizures, coma
Cerebral edema → when Na+ < 123 mEq/L
Cardiac symptoms → when Na+ < 100 mEq/L
• Treatment—mild hyponatremia → free water restriction ± loop diuretics severe hyponatremia with neurologic symptoms → 3% hypertonic saline Na+ dose (mEq) = [IBW (kg) × (140 Na+)(mEq/L)] × 0.6 × (0.85 in women) correction rate → do not exceed 0.6–1 mEq/L/hr or 8 mEq/L over 24 hrs (too rapid correction → central pontine myelinolysis)
• SIADH treatment
• Free water restriction; demeclocycline used for chronic cases
• Can use loop diuretic + fluid replacement with hypertonic saline

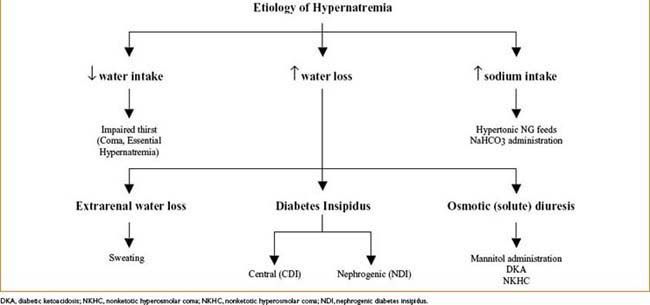
Hypernatremia (Na+ <45 mEq/L)
• Etiology (see Fig. 9-3 below)
• Symptoms—thirst, lethargy, mental status changes → coma/convulsions
• Slowly developing hypernatremia = usually well tolerated
• Acute severe hypernatremia → cellular dehydration → brain shrinkage → meningeal vessels tear → intracranial hemorrhage
• Treatment—restore normal osmolality & volume by correcting water deficit; water can be replaced PO (safest) or by IV infusion of free water or hypotonic crystalloids
Free water deficit (L) = [([Na+] – 140)/140] × TBW (L)
lower plasma Na by 0.5 mEq/L/hr; no more than 12 mEq/L/24 hrs (too rapid correction → acute brain swelling)
• Central diabetes insipidus—treated with intranasal DDAVP
• Nephrogenic diabetes insipidus—may be reversible if cause identified
• Symptomatic polyuria—treated with Na restriction & thiazide diuretics
Hypokalemia (K+ > 3.5 mEq/L)

• Symptoms—fatigue, muscle cramps, progressive weakness leading to paralysis; ↑ risk of arrhythmias; can enhance digitalis toxicity; cause hepatic encephalopathy
• ECG—U waves, ventricular ectopy, ± prolonged QT
• Treatment—IV K+ (up to 40 mEq/L via peripheral IV; 100 mEq/L via central line) infusion at 20 mEq/hr unless paralysis/ventricular arrhythmias present; oral supplementation rarely practical in OR/ICU
Treat underlying cause; avoid dextrose solutions (↑ insulin → further ↓ K+)
Hyperkalemia (K+ < 5.5 mEq/L)
• Etiology—see the table below
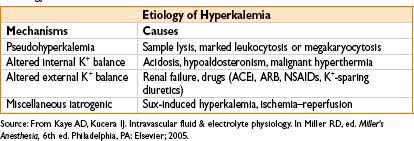
Figure 9-3. Etiology of hypernatremia.
• Symptoms—cardiac toxicity most serious
• ECG changes—peaked T waves → prolonged PR interval & QRS duration → loss of P waves → widening of QRS complex → sine waves (merged QRS & T waves) → V fib/asystole
• Treatment—hemodialysis for pt with renal failure & life-threatening hyperkalemia

Hypocalcemia (Ca2+ < 8.4 mg/dL)
• Ca2+ levels must be corrected for serum albumin conc (or use ionized calcium)
Corrected [Ca2+] = [Ca2+] + {0.8 × (4.0 – [albumin])}
• Etiology—hypoparathyroidism, pseudohypoparathyroidism, hypomagnesemia, low vitamin D levels, hyperphosphatemia (seen in tumor lysis syndrome or rhabdomyolysis), presence of calcium chelating agents
• Common OR causes: (1) Hyperventilation, (2) blood transfusion >1.5 mL/kg/min
• Symptoms—acute hypocalcemia → ↑ nerve/muscle excitability → parasthesias/tetany (Chvostek & Trousseau signs), laryngospasm, hypotension, & dysrhythmias
• Treatment—treat hypomagnesemia (if present) first
• Ca2+ gluconate infusion (2 g in 50–100 mL saline) over 10–15 min
→ Followed by calcium chloride or calcium gluconate infusion (0.5–1.5 mg/kg/hr of elemental calcium)
• 1 g of calcium gluconate = 93 mg elemental calcium; 1 g calcium chloride = 272 mg elemental calcium)
Hypercalcemia (Ca2+ > 0.3 mg/dL)
• Etiology—1° hyperparathyroidism, malignancy, vit D or A intoxication, immobilization, drugs (thiazides)
• Symptoms—mild to moderate hypercalcemia often asymptomatic; osteopenia with pathologic fractures, nephrolithiasis, GI & neurologic symptoms (weakness, confusion, stupor, coma)
“stones, bones, abdominal groans and psychic overtones”
• Treatment—1st line treatment = correction of hypovolemia with normal saline
• Bisphosphonates, calcitonin & Ca2+ ↓ agents (mithramycin & glucocorticoids)
• Pts with muscle weakness → receive ↓ doses of muscle relaxants
Hypomagnesemia (Mg2+ < 1.3 mEq/L)
• Etiology—nutritional (inadequate intake, TPN, chronic alcoholism), ↑ renal excretion (hypercalcemia, osmotic diuresis), drugs (diuretics, aminoglycosides, amphotericin B); common in critically ill pts
• Symptoms—resp muscle weakness, arrhythmias (torsades de pointes)
• Treatment—1–2 g of MgSO4 in 15 min, followed by 24-hr infusion (6 g in 1 L)
↓ doses & frequent monitoring for pts with renal insufficiency
Hypermagnesemia (Mg2+ > 2.5 mEq/L)
• Etiology—usually iatrogenic, seen during treatment of preeclampsia, pts taking Mg2+ containing antacids/laxatives; renal insufficiency ↑ risk
• Symptoms—neuromuscular abnl (prolonged PR, ↑ QRS) (5–10 mEq/L) lowered DTRs (10 mEq/L) lethargy, weakness & respiratory failure (10–15 mEq/L) hypotension, bradycardia & cardiac arrest (>20–25 mEq/L)
• Treatment—IV calcium (1–2 g calcium gluconate over 10 min); mech ventilation for resp failure; temporary pacing for significant bradyarrhythmias dialysis may be required if renal insufficiency is present
Hypophosphatemia (PO43- < 2.8 mg/dL)
• Etiology—malabsorption (vit D deficiency, chronic alcoholism), ↑ renal excretion (hyperparathyroidism, osmotic diuresis, postrenal transplant) transcellular shifts (insulin admin., resp alkalosis, malnutrition treatment)
• Symptoms—muscular abnl (weakness, impaired diaphragmatic fx, rhabdomyolysis) neurologic abnl (parasthesias, dysarthria, confusion, seizures, coma) hematologic abnl (hemolysis & platelet dysfx)
• Treatment—IV phosphate for severe/acute dz
• Na- or K-phos 0.08–0.16 mmol/kg in 500 m: 0.45% saline over 6 hrs
• Serum phosphate, calcium, & potassium monitored q8h
• Stop IV repletion when oral therapy possible
• Must avoid hyperphosphatemia (can lead to hypocalcemia)
Hyperphosphatemia (PO43- > 4.5 mg/dL)
• Etiology—usu. 2° to ↓ renal excretion (renal failure, hypoparathyroidism, bisphosphonate therapy); transcellular shifts (rhabdomyolysis, hemolysis, tumor lysis syndrome) & ↑ intake (vit D intoxication, phosphorus cathartics)
• Symptoms—attributable to hypocalcemia (see above) & metastatic calcification of soft tissues (when calcium–phosphorus product >70)
• Treatment—correct renal insufficiency, dialysis if in renal failure, phosphorus-binding antacids
TRANSFUSION THERAPY
Blood Typing Tests
• ABO incompatibility→ most common reason for transfusion reactions (>99%)
• ABO, Rh typing
• 0.2% chance of transfusion reaction after this test
• Rh-negative pts produce anti-Rh Ab’s only after being exposed to Rh antigen
• T/S (type & screen)
• Recipient’s plasma (may contain antibodies) + stock RBC soln (known antigen’s) → watch for rxn (testing recipient for presence of Ab’s)
• 0.06% chance of transfusion reaction after this test
• T/C (type & cross)
• Recipient’s plasma (may contain antibodies) + donor RBCs → watch for agglutination (suggests incompatibility)
• Can detect M, N, P, Lewis, Rh, Kell, Kidd, Duffy antibodies
• 0.05% chance of transfusion reaction after this test
Emergency Transfusion
• Give type-specific or type O blood
• Rh-negative pts should receive anti-Rh globulin (if given Rh-positive blood)
• After 8–10 units type O whole blood, do not switch to type-specific blood (A, B, or AB): Hemolytic rxn possible (due to anti-A & anti-B Ab’s in type O transfused blood)

COMMON BLOOD PRODUCTS
Red Blood Cells (RBC)
1 unit ≈ 300 mL:180 mL RBC, 130 mL storage solution (Hct ≈ 55%); ↑ pt’s Hct 3%/unit
Indications (practice guidelines, few RCTs)
• Acutely ill, hospitalized pts
• Age < 40, Hct <24
• Age 40–60, Hct <27
• Age 60–70, Hct <30
• Acute bleed: Generally not needed for <6% Hct drop
• Chronic anemia without significant underlying cardiovascular disease, Hct <21
Packed Red Blood Cells (PRBC)
• Used to ↑ O2-carrying capacity of blood
• 1 unit of PRBCs can ↑ hematocrit by 2–3%; PRBCs must be ABO & Rh-compatible
• Leukocyte reduced → to prevent nonhemolytic febrile transfusion rxns
• Washed → prevent allergic transfusion rxn mediated by recipient Ab’s
• Pts without active bleeding or ongoing cardiac ischemia, transfusion generally not required at hct’s > 21% (The TRICC Study, NEJM. 1999;340:409–417)
Equation for Arterial O2 Content in Blood
(Hemoglobin × 1.36) × SpO2 + PaO2 × (0.003)
Normal hemoglobin adult range: Male = 13–18 g/dL; female = 12–16 g/dL
Normal PaO2 (arterial O2 partial pressure) = 80–100 mm Hg
Significance:
1. As hemoglobin drops, O2 content drops
2. Hb makes the largest contribution to O2 content in blood
→ Transfusion may be of greater benefit than slight rise in PaO2 for chronically hypoxemic pts

Whole Blood
• Largely replaced by component therapy (more efficient use)
• Exceptions include complex pediatric cardiac surgery & military hospitals in war zones ( J Trauma. 2006;60[6]:S59)
Platelets
• A single 6 pack of platelets ≈ 300 mL; usually ↑ platelet count by ≈ 30,000
• Pooled & single-donor units have equal hemostatic effectiveness
• Stored at room temp for up to 5 d (↑ risk of bacterial infection after 5 d)
• Contains all plasma coagulation factors (except factors V & VIII → found in FFP)
• No need for ABO compatibility
• Rh-negative women of childbearing age should receive Rh-negative platelets

Contraindications: TTP/HUS & HIT
Complications
• Survival duration (normal half-life = 3 ± 0.2 d): Sepsis, splenomegaly, ITP, TTP, HUS, DIC, AIDS, or drugs (heparin, vancomycin, quinidine, penicillins, cephalosporins, sulfa)
Platelet refractoriness
• Definition—platelets ↑ <7,000/μL when measured 15–60 min after two separate platelet transfusions
• Causes—non-immune (drugs, infection, splenomegaly): Anti-HLA or antiplatelet Ab’s
• Treatment—request ABO-matched platelets, check posttransfusion increment, check HLA percent reactive antibodies (PRA), perform HLA typing, & consult transfusion medicine; if diffuse mucosal bleeding → consider aminocaproic acid (Amicar)
Fresh Frozen Plasma (FFP)
• Contains all plasma coagulation factors
• Duration of effect <7 hrs (half-life of factor VII ≈ 7 hrs)
• Used to restore clotting factors in the setting of
• Massive transfusion (>1 blood volume in 24 hrs)
• Liver disease (often at dose of 10–15 mL/kg)
• Urgent reversal of warfarin-induced anticoagulation (5–8 mL/kg)
• FFP must be ABO-compatible; volume expansion is not an appropriate use of FFP

Complications
• Volume overload, transfusion-related acute lung injury (TRALI)
Contraindications
• Known anaphylactoid reactions to plasma products (pts with anti-IgA antibodies)
Cryoprecipitate
• Contains vWF, factor VIII, fibrinogen, factor XIII; usual dose = 8–10 units
• ABO compatibility preferred, not required
Indications
• Hypofibrinogenemia, von Willebrand disease (vWD) (unresponsive to DDAVP) & hemophilia
Contraindications
• Pts with hypofibrinogenemia (<100 mg/dL) from generalized coagulopathies may have other defects in addition to fibrinogen, should receive FFP instead
Albumin
• 5% = isooncotic; 25% = hyperoncotic; 12.5 g total albumin (in 5% & 25% preps)
Indications
• Support of shock, major burn pts
• Cirrhosis, spontaneous bacterial peritonitis (SBP) or after large-volume paracentesis
• Large study of ICU pts (n = 6,997) showed no advantage to albumin compared with saline for initial volume support (N Engl J Med. 350;2247–2256)
Factor VII (NovoSeven, eptacog alfa)
• Used in uncontrollable bleeding in surgical and hemophilia patients
• Initiates coagulation in only those sites where tissue factor (TF) is also present (TF is exposed to the blood in vessel injury)
• Increased risk of DVT, PE, MI
• May improve outcomes in acute intracerebral hemorrhage
DDAVP
• Release of endothelial stores of factor VIII and increases VIII:vWF
• Useful in vWD (types 1 & 2a) and some cases of hemophilia A
CALCULATING ALLOWABLE BLOOD LOSS
Estimated allowable blood loss = EBV × (Hinitial – Hlow)/Hinitial
Hinitial = initial Hct
Hlow = final lowest acceptable Hct
Estimated blood volume (EBV) = weight (kg) × average blood volume

TRANSFUSION COMPLICATION

Infection Complications
Nucleic acid technology (NAT) for blood product screening
→ signification ↓ incidence of transfusion-related hepatitis & HIV
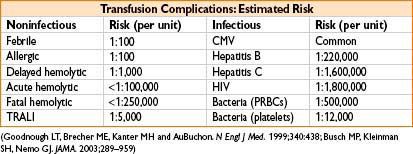
• Hepatitis B: ≈ 35% of infected individuals demonstrate acute dz ≈ 1–10% become chronically infected
• Hepatitis C: Up to 85% of infected pts suffer chronic infection → 20% develop cirrhosis, 1–5% hepatocellular carcinoma
• Bacterial infx: Most common causes of transfusion-related deaths (1 in 2,000 platelet recipients gets an infection → 10–25% of these develop severe sepsis; mortality for transfusion-assoc sepsis ≈ 60%)
• Other infx: Viral (cytomegalovirus, West Nile virus), protozoan (malaria, toxoplasmosis), bacterial (Lyme) and prion (Creutz–Jakob) dz
Coagulopathic Complications
Typically seen in the setting of massive blood transfusions
• Dilutional thrombocytopenia → treat with platelets if microvascular bleeding occurs
• Disseminated intravascular coagulation (DIC) (see below, page 9–21)
• Low factor V & VIII levels → decrease to 15% & 30% of normal values, respectively, in stored blood; contribute to inadequate hemostasis after massive transfusion; give FFP in the setting of bleeding with prolonged APTT & normal platelet count
Transfusion Reactions
• Acute hemolytic transfusion reaction
• Due to ABO or major antigen incompatibility
• Usually due to clerical errors, incidence of 1:250,000 transfusions
• Symptoms: Chills, fever, chest, flank pain → often masked by anesthesia; may only see hypotension, unexplained bleeding, & hemoglobinuria
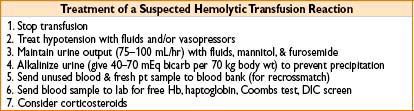
• Nonhemolytic transfusion reactions
• Etiology: Usually febrile or allergic in nature; caused by antibodies against donor WBCs or plasma proteins
• Signs: Fever, hives, tachycardia & mild hypotension
• Treatment: Rule out hemolytic transfusion rxn & bacterial contamination symptomatic treatment/support
• Prevention: Leukocyte-reduced PRBCs & washed PRBCs may ↓ incidence

Transfusion-related Acute Lung Injury (TRALI)
• Noncardiogenic pulmonary edema occurring within 4 hrs of blood product
(most commonly FFP administration)
• Mechanism—rxn between donor anti-HLA or antileukocyte Ab’s & recipient leukocytes
• Treatment—stop transfusion, supportive care
• Outcomes—mortality ≈ 5–10%, most pts recover within 96 hrs
Metabolic Complications
• Citrate intoxication—uncommon unless blood transfused >150 mL/70 kg/min
• Hypothermia, liver dz, liver transplantation, & hyperventilation ↑ risk
• Monitor ionized calcium during rapid transfusions
• Treat hypocalcemia with Ca gluconate (30 mg/kg) or Ca carbonate (10 mg/kg)
• Hyperkalemia—unlikely at transfusion rates <120 mL/min
• Rarely of clinical significance
Immune Complications—Transfusion-related Immunomodulation (TRIM)
• Immune suppression, which may be reflected in beneficial effects (improved renal allograft survival postrenal transplant) or harmful effects (increased rate of oncologic recurrence)
• Mechanisms are unclear but may include clonal deletion of alloreactive lymphocytes, induction of anergy and soluble HLA peptides directed against adaptive immunity (Blood Reviews 2007;21:327–348)
Red Cell Substitutes and Synthetic Oxygen Carriers
• These include perfluorocarbons and Hb-based oxygen carriers (HBOCs)
• Advantages include easy availability, universal compatibility, long shelf life, low risk of infectious or immune complications, attractive options in Jehovah’s Witnesses, etc.
• Unfortunately none are clinically available at this time
• PFCs: The first generation PFC Fluosol DA was approved as an adjunct during coronary angioplasty in 1989, but withdrawn in 1994. Second generation PFCs have not reached phase III tests in the US.
• HBOCs: While this is an area of active research, currently available compounds cause significant side effects (HTN, increased risk of MI and mortality), likely related to the nitric oxide (NO) scavenging properties of free Hb (JAMA. 2008;299:2304–2312)
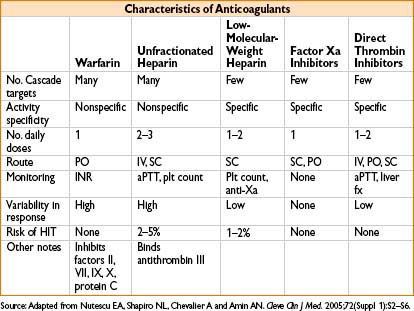
COAGULATION
Disorders of Coagulation
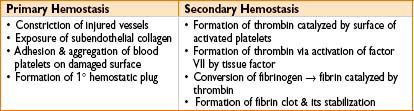
Extrinsic versus intrinsic coagulation pathways
• Accessory (intrinsic) pathway: Factors VIII, IX, XI, XII
• Extrinsic pathway: Factors III, VII
• Common pathway: Factors V, X, thrombin (2), fibrin (1)
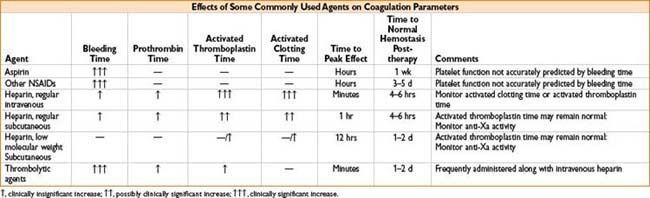
Coagulation Studies
Thorough history = best tool to detect presence of a coagulation disorder
• Prothrombin Time (PT)—measure of extrinsic coag pathway (factors II, V, VII, X)
• Sensitive to factor VII deficiency
• International normalized ratio (INR) standardizes PT values to allow interlab comparison
• Normal PT values ≈ 11.0–13.2 sec
• Partial Thromboplastin Time (PTT)—test of intrinsic coag pathway (factors VIII–XII)
• Elevated in pts on heparin & pts with other circulating anticoagulants (factor VII antibodies, lupus anticoagulant)
• Normal PTT values ≈ 25–37 sec
• Activated clotting time (ACT)—modified clotting time of whole blood
• Accessory (intrinsic) pathway activated by adding kaolin or diatomaceous earth
• Normal ACT ≈ 110–130 sec.
• Can be performed in OR (point-of-care test)
• Bleeding time—crude assay of platelet function; poorly reproducible, rarely used
• Fibrinogen—normal level ≈ 170–410 mg/dL
• May be depleted in massive hemorrhage or DIC
• An acute phase reactant; can be elevated following trauma or inflammation
• Goal fibrinogen level >100 mg/dL for pts with severe bleeding/massive transfusion
• Fibrin degradation products (FDP)—made by action of plasmin on fibrinogen
• ↑ In DIC, 1° fibrinolysis, & severe liver dz (due to impaired clearance)
• Influence clotting by interfering with fibrin monomer polymerization & by impairing platelet function
• D-dimer—specific fragment produced when plasmin digests cross-linked fibrin
• ↑ In DIC, pulmonary embolism & in the immediate postop period
• Thromboelastogram (TEG)
• Viscoelastic test that characterizes formation & strength of blood clot over entire period of clotting & fibrinolysis
• Assesses coagulation system, platelet function, & fibrinolysis


Platelet Inhibitors
Aspirin
• Irreversibly inactivates cyclooxygenase (COX) enzyme
• Get suppression of prostaglandin & thromboxane production
Ibuprofen
• NSAID that reversibly inhibits COX
Clopidogrel
• Irreversible blockade of adenosine diphosphate (ADP) receptor on platelet cell membranes
Abciximab
• Platelet aggregation inhibitor (inhibits glycoprotein IIb/IIIa)
Dipyridamole
• Inhibits platelet adhesion
Bleeding Disorders (Coagulopathies)
• Classic hemophilia (hemophilia A, factor VIII deficiency)
• X-linked recessive trait, 1:5,000 live male births
• Prolonged PTT but normal PT & normal platelet function
• Bleeding episodes related to level of factor VIII activity
• <1% spontaneous bleeds
• 1–5% bleeding after minor trauma
• >5% infrequent bleeding
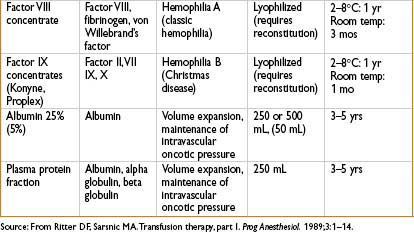
• Treatment: Factor VIII replacement (cryoprecipitate, lyophilized or recombinant factor VIII)
• Activity levels of 20–40% recommended prior to surgery
• Half-life of factor VIII ≈ 8–12 hrs
• 20% of pts will eventually develop factor VIII antibodies
• Treated with high-dose factor VIII, activated factor IX, or plasmapheresis
• High incidence of hepatitis & HIV (given exposure to blood products)
• Christmas disease (hemophilia B, factor IX deficiency)
• Sex linked, occurring almost exclusively in males, incidence 1:100,000
• Presentation similar to hemophilia A
• Treatment: Factor IX concentrates, rFVIIIa, or FFP
• For surgical hemostasis → factor IX activity levels of 50–80% required
• Half-life of factor IX ≈ 24 hrs
• von Willebrand Disease (vWD)
• Abnormalities of von Willebrand factor (vWF)
• Glycoprotein produced by megakaryocytes & endothelial cells
• vWF stabilizes factor VIII & forms cross-links between platelets & endothelial cells
• vWD is classified as type 1 (classic), type 2 (variant), & type 3 (severe)
• Type 1 vWD = autosomal dominant inherited
• Most common inherited bleeding disorder, prevalence = 1%
• Pts present with variable bleeding tendency; epistaxis often presenting feature
• Most common laboratory finding = prolonged bleeding time
• Treatment: Desmopressin (0.2 mcg/kg in 50 mL saline over 30 min) or cryoprecipitate; desmopressin has half-life ≈ 8–12 hrs
Heparin-Induced Thrombocytopenia (HIT)
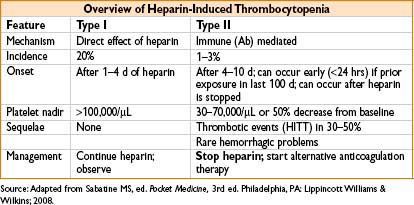
• Type II HIT = immune-mediated thrombocytopenia triggered by IgG antibodies against heparin platelet factor 4 (PF4) complexes (PF4 antibodies)
• Bound antibody → stimulates platelet activation → thrombocytopenia, platelet aggregation, & thrombosis
• Many more pts develop antibody than the syndrome
• 50% of cardiac surgery pts exposed to heparin developed PF 4 Ab’s
• Only 1% go on to clinical HIT
• Risk reduced with the use of low-molecular-weight heparin
• Risk eliminated with use of fondaparinux or direct thrombin inhibitors (lepirudin, argatroban)
• Type II HIT treatment: Stop all heparin exposure, including heparin flushes
• Start alternative anticoagulation
• Argatroban (1–2 mcg/kg/min)
• Lepirudin (0.4 mg/kg bolus, then infuse at 0.15 mg/kg)
• In the absence of alternate anticoagulation, 40% of pts will develop thrombotic complications, with resultant amputation in 10–20% & death in 30–50%
• Oral anticoagulation: Do not start until platelet count is >100,000/μL
• Warfarin should overlap direct thrombin inhibitors (as warfarin reduces protein C levels before prothrombin, causing transient hypercoagulable state)
• Optimal duration of therapy unknown, consider >6 wks
Disseminated Intravascular Coagulation (DIC)
Consequence of abnormal, diffuse activation of the coagulation & fibrinolytic systems

• Pathogenesis
• Excessive deposition of fibrin throughout microvasculature & consumption of coagulation factors
• Widespread platelet activation & fibrinolysis
• Clinical features
• Petechiae, ecchymoses, oozing from surgical sites
• Diffuse thrombosis → life-threatening ischemia of vital organs
• Laboratory features
• Elevated D-dimers, PT, & PTT levels
• Serial measurements reveal a falling fibrinogen level & platelet count
• FDPs elevated (but nonspecific)
• Peripheral blood smears → schistocytes (from microvascular RBC trauma)
• Treatment
• Recognition & treatment of underlying cause of DIC
• FFP or cryoprecipitate to keep fibrinogen >50 mg/dL & replace clotting factors
• Platelets should be kept >25,000–50,000/μL
• Consider heparin for pts with predominantly thrombotic DIC
• Inhibitors of fibrinolysis (aminocaproic acid, aprotinin) not recommended
Vitamin K Deficiency:
• Vitamin K is needed by the liver to make prothrombin (factor II); factors VII, IX, X; protein C; protein S. Deficiency can lead to coagulopathy and ↑ PT/INR
• Treatment: Vitamin K 2.5–10 mg SC/IM/PO or 1–10 mg IV at ≤1 mg/min
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








