CHAPTER 43 Echocardiography in the CICU
Advances in echo technology and the ability to digitally store data enable acquisition of accurate information that can easily be displayed and compared with prior studies for serial comparison. Echocardiography has increasing utility in guiding management of a wide spectrum of cardiovascular disorders. It has been shown to influence therapeutic decision making in critically ill patients and provide supplemental information beyond that obtained by pulmonary artery catheterization.1,2
This section is divided into the following categories:
Evaluation of Patients with Chest Pain
Each year 6 million patients in the United States come to the emergency room with chest pain.3 The sensitivity of electrocardiography in diagnosing acute myocardial infarction (MI) is relatively low.4 Detection of regional wall motion abnormalities by echocardiography is a very sensitive method for diagnosing an acute MI. If no regional wall motion abnormalities are present during chest pain, ischemic risk is very low.5,6,7 Although not approved by the U.S. Food and Drug Administration for this indication, numerous studies have demonstrated the incremental value of perfusion echo in identifying the presence of ischemia. Kaul and colleagues6 compared contrast echocardiography with a modified Thrombolysis In Myocardial Infarction (mTIMI) score for triage of nearly 1000 patients having chest pain and nondiagnostic ECG before troponin levels became available. Both regional myocardial function and perfusion were analyzed by rest contrast echocardiography and related to early (in the first 24 hours), intermediate (at 30 days), and late (1 year) events. Only 0.4% of patients with normal regional function had a primary event consistent with an excellent negative predictive value of contrast echo. Contrast echocardiography was able to classify patients with an intermediate mTIMI score into low and high risk for acute coronary syndrome (ACS). When the troponin level was not available, the mTIMI score failed to identify approximately 4% of patients with ACS, consistent with previous data. The regional function provided incremental prognostic information beyond the mTIMI score for predicting intermediate and late events. Myocardial perfusion provided additional prognostic information beyond the mTIMI score and regional function in patient with abnormal regional function. The complete TIMI score did not provide incremental prognostic benefit when compared with the combination of mTIMI, regional function, and myocardial perfusion in the prediction of early and intermediate events. Therefore, in patients with chest pain and nondiagnostic ECG, rapid identification of regional function and myocardial perfusion help to risk stratify patients even before troponin levels are known.
Echocardiography in Patients with Acute Coronary Syndrome
Evaluation of Left Ventricular Function
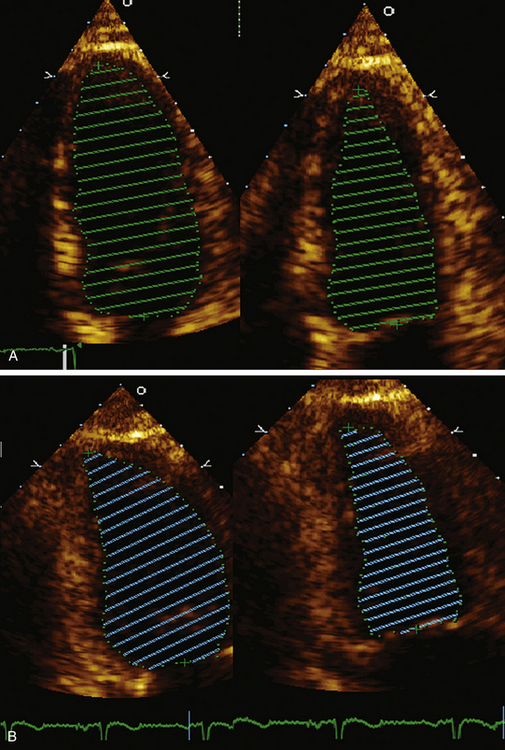
Accurate assessment of left ventricular systolic function and ejection fraction are critical in patients with myocardial infarction (MI) since further interventions, such as placement of an AICD or BiV pacemaker, require a certain ejection fraction (EF) as an indication. In addition, LV ejection fraction provides important prognostic information in patients with ST elevation myocardial infarction (STEMI). LV function has been shown to be an important determinant of mortality and subsequent development of congestive heart failure, and development of ventricular arrhythmias and sudden cardiac death.8–11 Figure 43-1 demonstrates the recommended method for calculation of EF by the American Society of Echocardiography.
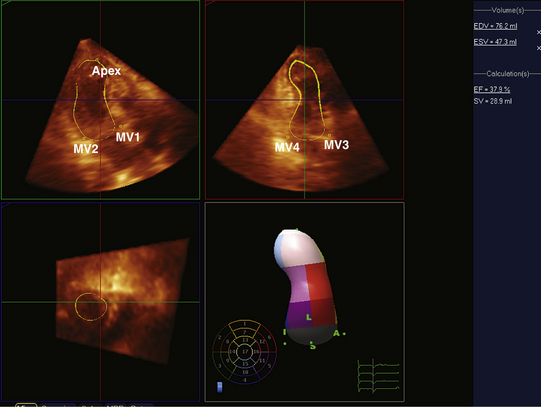
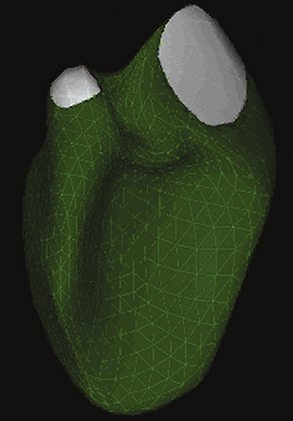
In patients in whom accurate quantification of systolic function cannot be obtained with 2D echo, new techniques such as 3D echo can be used. Multiple studies have confirmed the accuracy and feasibility of a 3D echocardiogram for assessment of LV volumes and function,12–15 and demonstrated good correlation between these parameters and those obtained by MRI.16–18 Because a data set comprises the entire LV volume, the greatest advantage of 3D echo over 2D echo is that there is no need for any assumption on the shape of the ventricle, which is required for volume calculation obtained from two planes only. As a result volume calculations using 3D echo are more accurate. This is especially true in patients in whom the geometry and shape of the LV is distorted secondary to MI and the presence of wall motion abnormalities and or an aneurysm. In these patients, direct measurements of full volume acquired by 3D will result in a more accurate analysis of structure and function than by making erroneous assumption using 2D data. Advances in technology now enable 3D data acquisition to be performed at the bedside. (Fig. 43-2 demonstrates 3D reconstruction of the LV). If image quality is limited, acquisition can be combined with infusion of contrast to improve the delineation of the endocardial border.
LV Aneurysm
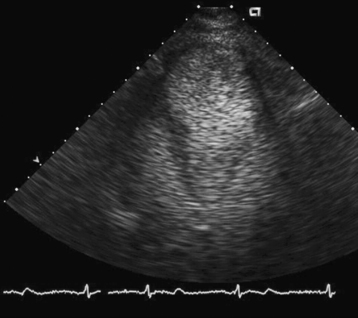
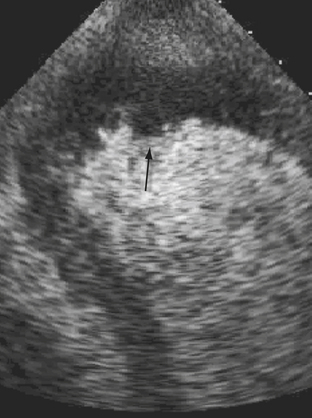
Aneurysm is defined as a discontinuity in the left ventricular contour that is present during both systole and diastole. It is formed when normal myocardium is replaced by necrotic myocardium and fibrous scar tissue, and is therefore a potential complication of transmural infarction. Mechanically, the aneurysm is a reservoir of blood that is not ejected, thus reducing the overall stroke volume, which could lead to the development of heart failure. Other potential consequences of the presence of an aneurysm are the development of a clot due to stagnation of blood and intractable arrhythmias due to the presence of scar tissue. Heart failure is a common indication for surgical resection of the aneurysm. Patients with ejection fraction of 35% or more generated by contractility of the basal portion of the ventricle, as measured by the basal fractional shortening, derive more benefit from aneurysm resection.19 Echocardiography plays a major role defining the size and location of an aneurysm and excluding the presence of a clot. It is instrumental in distinguishing it from a pseudoaneurysm by demonstrating the presence of a wide neck communicating with the ventricular cavity. Use of contrast echocardiography can clearly delineate the aneurysm better (Fig. 43-3). Three-dimensional echocardiography has a role in accurate determination of EF in these patients with abnormal geometry as outlined in detail previously.
Apical Clot
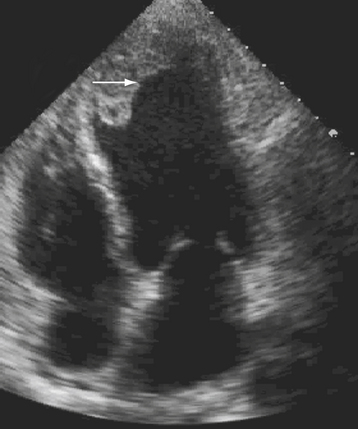
Left ventricular thrombus usually occurs in anteroapical infarction although it can infrequently be seen in inferior myocardial infarction as well (Fig. 43-4). A thrombus can be mobile, pedunculated, or laminar in nature. It occurs in areas of extensive regional wall motion abnormalities, where stagnation of flow is expected, or within an aneurysm. Although the majority of clots form at least 72 hours after the onset of MI, it can occasionally be seen much earlier and emphasis should therefore be made to exclude their presence on any echo performed in a post–MI patient. Identification of a thrombus is important because these patients require anticoagulation therapy for at least 6 months to reduce risk of embolic events. Two-dimensional echo can be repeated to assess resolution of a thrombus. The use of echo contrast has significantly increased the sensitivity of echo for detection of small clots, which appear as filling defects.20 Figure 43-5.
Exclusion of Right Ventricular (RV) Involvement
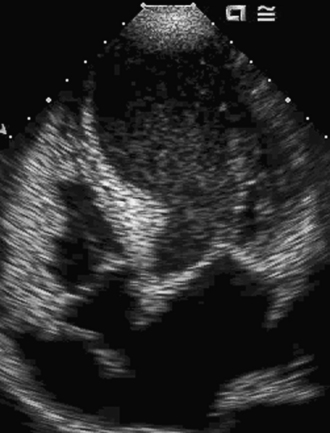
The incidence of right ventricular infarction in association with left ventricular myocardial infarction ranges from 14% to 84% and is dependent on the population studied and the pathologic criteria.21 Right ventricular (RV) infarction usually occurs in association with inferior wall infarcts, less commonly with anterior wall infarcts, and rarely in isolation. The clinical triad characteristic of RV infarcts is: hypotension, clear lungs, and elevated jugular venous pressure. The Kussmaul sign (an increase in jugular venous pressure with inspiration) is a highly sensitive and specific clinical finding for the diagnosis of RV infarction. These patients have normal LV filling pressures, but elevated right atrial and ventricular filling pressures. ST elevation seen on right-sided ECG leads (V3R, V4R, and V5R) is very suggestive of RV involvement. Echocardiography identifies depression of right ventricular systolic function with segmental wall motion abnormalities, and dilation of the right ventricle. Abnormal interventricular septal motion is also seen due to increased right ventricular end-diastolic pressure. The parasternal short axis view has the highest sensitivity (82%) with specificity ranging between 62% to 93% for hemodynamically significant right ventricular infarction.22 Interatrial septal bowing toward the left atrium, indicative of an increased right atrial-left atrial pressure gradient, has been shown to be an important prognostic marker in right ventricular infarction. This finding is associated with hypotension, heart block, and increased mortality.23 Recently 3D echocardiographic assessment of RV volumes in healthy adults has been shown to be reproducible, accurate, and closely correlated with the assessment of RV volumes using cardiac MRI.24 Given the complex structure of the RV, 3D echo may prove to be the preferred method for accurate assessment of RV structure and function, especially in patients with dilated RV and reduced function Figure 43-6. The treatment of right ventricular infarction includes aggressive maintenance of RV preload, reduction of RV afterload, inotropic support, and early reperfusion.
Ischemic Mitral Regurgitation (MR)
Myocardial infarctions (MIs) often trigger a remodeling process that leads to gross ventricular distortion, contractile dysfunction of normally perfused myocardium, symptomatic congestive heart failure (CHF), and premature death.25 Depending on size, location, and transmurality of the infarct, the remodeling process may be associated with the development of ischemic mitral regurgitation (IMR).26 Mild degrees of mitral regurgitation after acute MI portend a substantially increased risk of cardiovascular mortality within 5 years, even in patients who do not initially have signs of overt CHF.27 Echocardiographic features include severe segmental hypokinesis/akinesis predominantly of the inferior wall, tethering of the mitral valve leaflets, and inadequate coaptation of the valve leaflets associated with mitral regurgitation28 Figure 43-7. It is critical to exclude the presence of a significant degree of ischemic MR by performing a transthoracic echocardiogram (TTE), followed by a transesophageal echocardiogram (TEE) as needed, particularly in those who are candidates for surgical revascularization since it has been shown that CABG alone does not reduce the severity of MR.29 Alternative surgical and percutaneous approaches to these patients using repair and placement of devices in an attempt to restore the original shape of the annulus are now being explored.29–33
Innovative 3D reconstruction software have been developed recently that allow accurate evaluation of the mitral annulus. The utility of this 3D data in guiding interventions still need to be determined.34
LVOT Obstruction
Recent case reports have described dynamic left ventricular outflow tract obstruction in the setting of anteroapical infarction (Fig. 43-8). In this instance, the basal portions of the myocardium are hyperdynamic compensate for the reduced stroke volume, causing systolic anterior motion of the mitral valve. Both these features can easily be recognized on TTE. This entity should be suspected in a patient having hypotension refractory to inotropes and an intra-aortic balloon pump. Since the LVOT obstruction is dynamic and caused by the hyperdynamic contractility of the basal segments, inotropic medications would worsen the hypotension and should be avoided. Similar mechanism of LVOT obstruction can also be seen in patients with left ventricular apical ballooning syndrome, also known as Takotsubo cardiomyopathy35,36 (Fig. 43-9).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree