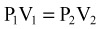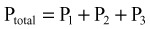133 Dysbarisms, Dive Injuries, and Decompression Illness
• Decompression illness describes diving-related injuries, including decompression sickness and arterial gas embolism.
• Any neurologic symptoms that occur in a diver immediately or soon after resurfacing are highly suspicious for arterial gas embolism and require urgent medical attention.
• A thorough neurologic examination is crucial with any suspicion of decompression illness.
• Dive injuries are best differentiated into the following categories: disorders of descent, disorders at depth, disorders of ascent, and disorders that occur after resurfacing.
• Decompression illness is treated on the scene by rapid implementation of high-flow oxygen and intravenous hydration; definitive treatment is recompression with hyperbaric oxygen therapy.
• One should delay flying after diving for at least 12 hours following a single no-decompression dive and at least 24 hours for dives involving decompression stops.
• Consultation with a diving physician or the Divers Alert Network (or both) is needed for a patient with any symptoms associated with recent diving.
Epidemiology
Dysbarisms refer to the pathophysiologic effects of changes in ambient (surrounding) pressure on the body. Decompression illness (DCI) includes decompression sickness (DCS or “the bends”) and arterial gas embolism (AGE). DCI occurs during or after ascent (decompression) when dissolved gases come out of solution, form bubbles, and then become lodged in various tissues (instead of being filtered by the lungs). Diagnosis of DCI in the emergency department (ED) is vital because delayed treatment or missed cases can have permanent sequelae (Box 133.1).
With the advent of “extreme sports” that involve water contact, sport diving, and the increasing number of people engaging in breath-hold diving, a sharp increase in diving injuries has been seen in EDs (Box 133.2). Deaths from breath-hold diving alone have almost doubled in the last 5 years, thus illustrating the potential for injury associated with this form of diving.1 With acute DCI, rapid assessment and treatment are the foundation of management. Three keys to successful ED treatment are having a high index of suspicion (DCI may have nonspecific findings), performing a thorough neurologic examination, and obtaining hyperbaric medicine consultation when DCI is suspected.
Box 133.2
Fatality Statistics for Diving
Deaths: 120 diving fatalities in the United States and Canada (2007)
Death analyses: men (85%); median age 50 years (men) and 43 years (women)
Obesity: 76% of deaths involved overweight or obese divers
Causes of death: drowning (86%); acute heart condition (9%); arterial gas embolism (3%); decompression sickness (1%)
Activities: 66% of deaths during pleasure of sight-seeing diving
Data from Divers Alert Network. DAN report on decompression illness, diving fatalities and project dive exploration, 2008. Available at www.diversalertnetwork.org/.
Pathophysiology
Bubble Physiology
Knowledge of bubble mechanics and the effects of bubbles in various tissues is critical to understanding the pathophysiology and treatment of DCI. Venous bubbles are not usually problematic because the lungs can filter large gas loads. Bubbles have damaging effects when they remain within tissues or embolize. Bubbles can pass from the venous circulation to the arterial circulation via a right-to-left shunt (patent foramen ovale or arteriovenous malformation). Bubbles can grow from “nucleation sites” within body tissues, such as the joint spaces, tendon sheaths, periarticular sheaths, and peripheral nerves.2 Once inside these areas, bubbles can act as emboli and block perfusion of distal tissues or act as foreign bodies with resultant vascular damage through activation of the inflammatory and clotting cascades. Interestingly, scientists are now evaluating a possible biologic marker of DCI. As gas emboli within the circulation induce decompression stress, endothelial cells release microparticles in response to cellular activation or cell death. These microparticles may, in the future, reflect a biologic marker of decompression stress that can be used to gauge the extent of disease, efficacy of treatment, or prophylaxis.3
Principles of Gas Laws and Dysbarism
Boyle’s Law
Usually, as a diver descends deeper, the surrounding pressure increases in linear fashion. Water pressure at the surface is referenced as 1 ATA. In general, for every 33 fsw that the diver descends, there is an increase in pressure of 1 ATA. Therefore, a descent to 33 fsw would equal 2 ATA, a descent to 66 fsw would equal 3 ATA, and so on. Furthermore, for the first 33 fsw descended, the volume of gas present is reduced by half the original amount of gas at the surface. At 66 fsw, the volume is one third the original volume. The greatest changes in volume occur closest to the water surface and represent a significant vulnerability to injury (Fig. 133.1).
Presenting Signs and Symptoms
Barotrauma
Barotrauma is sustained from failure to equalize the pressure of an air-containing space with that of the surrounding environment. The most common examples of barotrauma occur during air travel and scuba diving.1,4 Barotrauma occurs only in gas-containing (compressible) body spaces. More than 95% of the body is composed of water (incompressible). Typical gas-filled spaces include the sinuses, middle and inner ears, air-filled areas within carious or filled teeth, and hollow viscous organs such as the intestines and lungs. Barotrauma incurred during descent is called a “squeeze.” Barotrauma incurred during ascent is called a “reverse squeeze,” “reverse block,” or expansion injury.
Differential Diagnosis and Medical Decision Making
Table 133.1 lists the differential diagnosis for dive injuries based on the time of onset of symptoms.
Table 133.1 Differential Diagnosis of Dive Injuries Based on the Onset of Symptoms
| SYMPTOM ONSET | INJURIES TO CONSIDER |
|---|---|
| Descent | |
| Bottom | |
| Ascent | |
| 15 min after resurfacing | |
| 15 min to 24 hr after resurfacing |
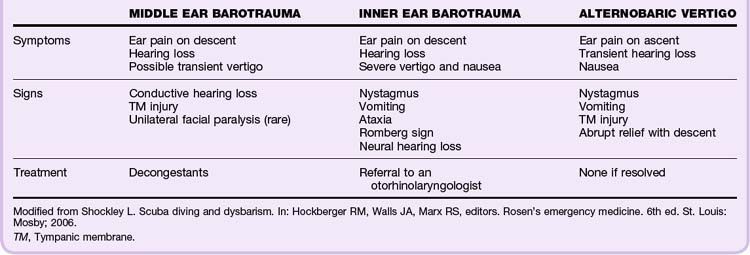
Ear Barotrauma
With an intact tympanic membrane (TM), the only communication for equilibration of pressure between the middle ear and the ambient atmosphere is through the eustachian tube (ET).5 Divers typically perform Valsalva maneuvers during decent to equalize pressure in the middle ear. Failure to equalize leads to pain and damage from injury to the middle or inner ear and results in TM edema, rupture, or hemorrhage, as well as rupture of the oval or round window (may lead to a perilymphatic fistula).5 Table 133.2 summarizes the types of ear barotrauma.