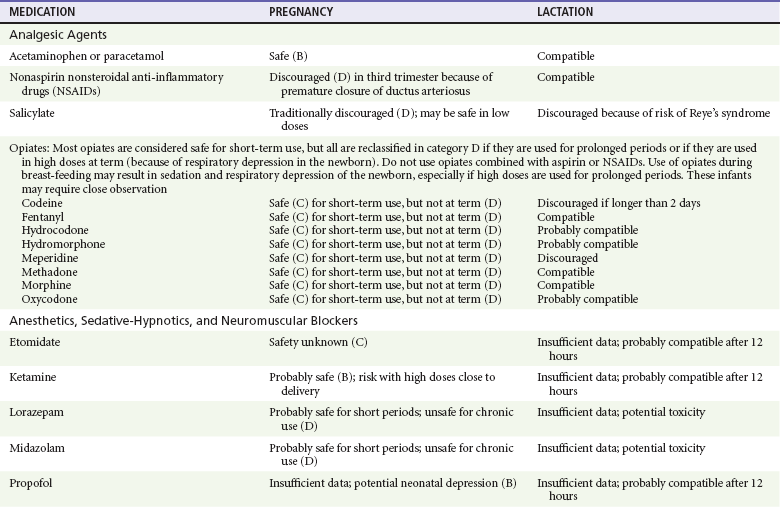
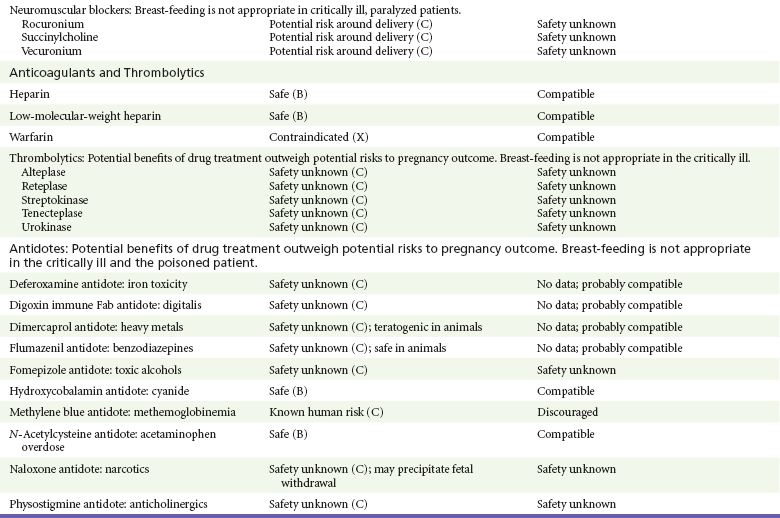
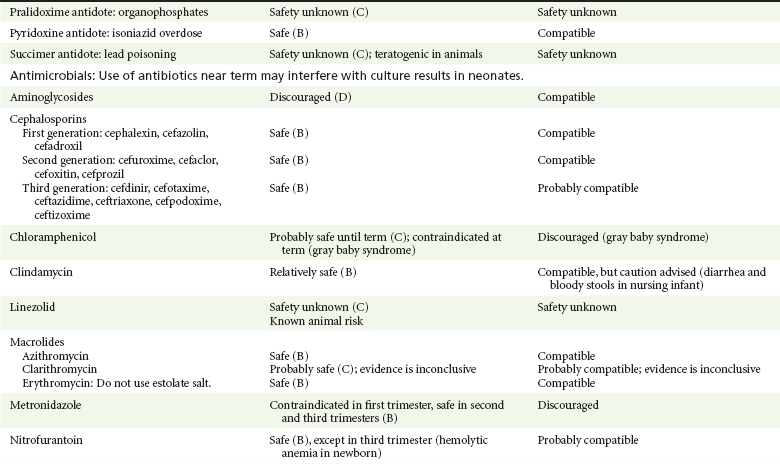
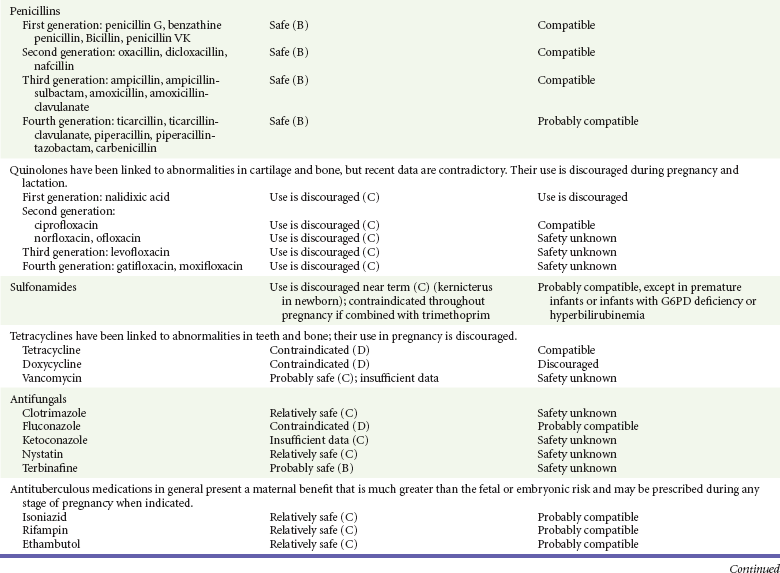
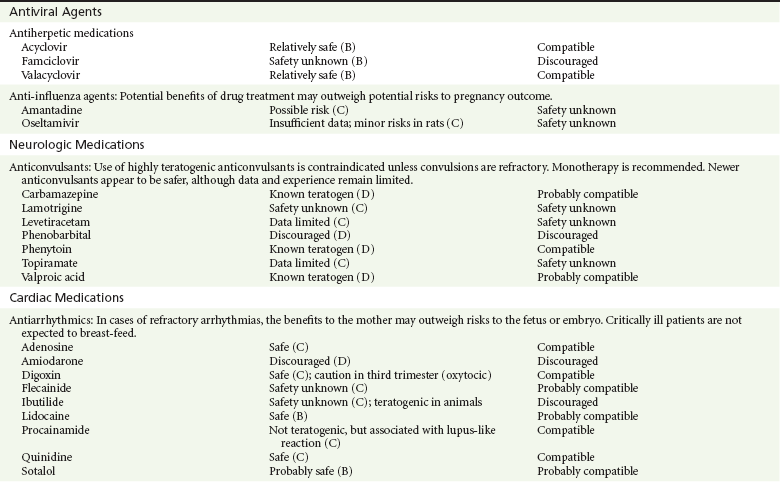
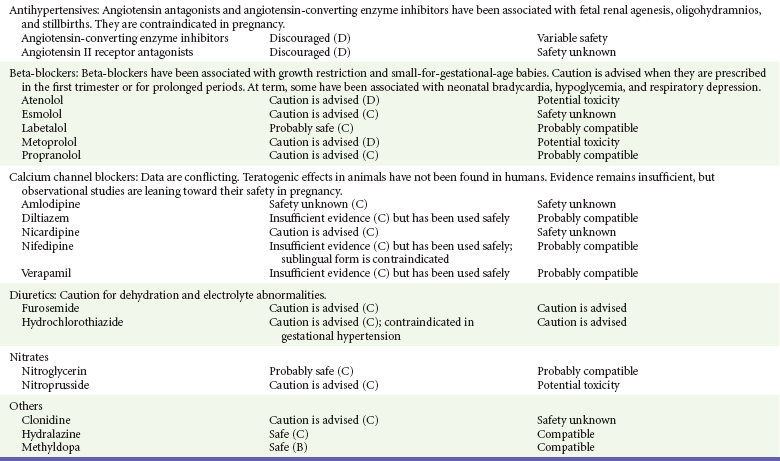
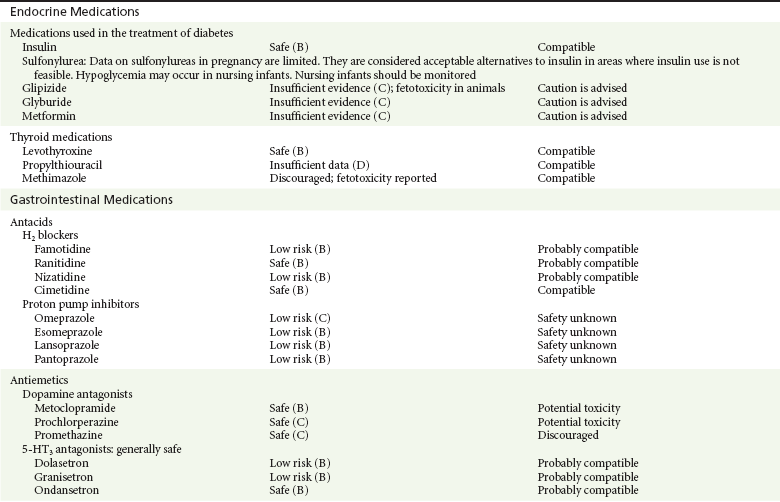
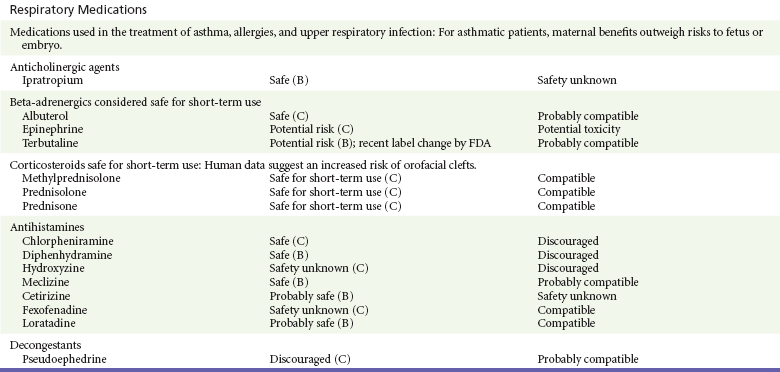
Chapter 180 Major birth defects affect 3 to 5% of all live births.1 Most are of unknown etiology, but 1 to 3% of these are thought to be due to pharmaceutical or environmental agents.1,2 A teratogen is any chemical, pharmacologic, environmental, or mechanical agent that can cause deviant or disruptive development of the conceptus.1,2 Included in this definition are functional impairment, growth restriction, and congenital malformations.2 These may range from subtle neurobehavioral effects to devastating physiologic effects and physical deformities, including fetal death.1–3 Why one pregnancy would be affected and not another remains to be elucidated. Highly teratogenic medications seem to be few in number, estimated at well below 50 agents (Box 180-1).3,4 The process of establishing teratogenicity is tedious and often flawed. Animal research, although valuable in determining risk initially, is not always applicable to humans,1,2,5 and controlled prospective human studies are generally not performed for ethical reasons. As a result, much of our current knowledge on teratogenicity has been derived from case reports, case-control studies, or cohort studies, which are inherently weak in establishing a causal relationship.1,2,5,6 These reports are often complicated by a multitude of confounding factors, making the determination of a causal link between a specific exposure and malformations difficult. The genetic background of the fetus, timing and duration of the exposure, environmental factors, multiple exposures, nutritional deficits, maternal illness, and illicit drug use all contribute to the outcome of pregnancy.1,2,5,6 Large population studies are needed to understand the connection between the outcome of a pregnancy and an associated in utero exposure.6 Finally, as in the case of diethylstilbestrol, teratogenicity may not be apparent for years after birth. To aid physicians in determining the teratogenic potential of a particular medication, the U.S. Food and Drug Administration (FDA) assigns one of five letters, A, B, C, D, and X, to the drug, depending on the strength of evidence for its safety or teratogenicity (Box 180-2). This classification system has been criticized as overly simplistic and perhaps inaccurate.1,2,5–7 Many clinicians believe that the classification system conveys the incorrect impression that there is a gradation of reproductive risk from exposure across categories (i.e., that risk increases from A to B to C to D to X) and that the drugs within a given category present similar reproductive risks.7 Yet more than 90% of newly introduced drugs in the United States are assigned to class C, an undetermined teratogenic risk.8 The FDA has acknowledged these problems, and in 2008 it proposed new rules for drug labeling during pregnancy. The proposed rules recommend giving a narrative description of the risks and the likelihood of developmental abnormalities and are awaiting the final stages of clearance.9 Currently, a number of clinical teratology resources that assign risk are available online. These include TERIS, REPROTOX, and Micromedex REPRORISK (Shepard’s Catalog of Teratogenic Agents). Drugs may affect the fetus through a variety of mechanisms. Some alter the availability of substrates, such as vitamins, glucose, oxygen, and amino acids, needed for normal nutrition and growth.1–3 Others directly affect cellular growth and differentiation. The age at which fetal exposure occurs is crucial in determining its impact on the pregnancy. The fetus is most vulnerable to toxic insults during the time of organogenesis (days 21-56 of fetal life).1–3 Exposure during this period may result in major anatomic defects. Exposure after the period of organogenesis may affect the growth and development of the fetus.1–3 Functional development of the central nervous system (CNS) is affected when it is exposed to a CNS teratogen during the 10th to 17th weeks of pregnancy.1–3 Drug transfer across the placenta occurs most commonly by simple passive diffusion or by protein transport.1–3,10 A thin layer of trophoblastic cells is all that separates maternal from fetal circulation.1–3,10 The degree to which a drug gains access to fetal circulation depends on molecular size, ionic state, lipid solubility, and extent of protein binding. Drugs with a molecular mass of less than 5 kDa readily diffuse.1–3,10 Anionic forms diffuse through the lipid layer more readily than ionized forms.1–3,10 Free drug diffuses more readily than a drug that is bound to plasma proteins.1–3,10 Because fetal pH is slightly more alkalotic than maternal pH, weak organic acids (e.g., salicylate) may become ion trapped in the fetal circulation, increasing fetal exposure.1–3,10 For the most part, drugs and substances that are ingested or injected by the mother diffuse passively into milk and then back into the maternal circulation for excretion.11 The amount of drug diffusing into milk depends on many factors. Lipid-soluble and nonionic substances diffuse more readily, and highly protein bound substances diffuse less readily.11 Whether a substance is concentrated in maternal milk or not, the neonate generally is able to detoxify it with no adverse effects, and only a few drugs pose a serious danger to a breast-feeding infant.11–13 The interruption of breast-feeding should not be advocated except in rare situations of known drug toxicity to the infant and in all cases of maternal critical illness.11,12 Table 180-1 summarizes the compatibility of medications and their effects in pregnancy and lactation. Acetaminophen (paracetamol) is widely used during pregnancy and has not been associated with congenital malformations.13–15 In fact, in a population-based case-control study, acetaminophen was associated with a decreased risk of certain craniofacial malformations when it was used for febrile illnesses in the first trimester.13,15 Acetaminophen is safe during lactation because only a small amount is excreted into breast milk, and the amount involved is tolerated by the neonate’s sulfhydration pathway.11–13 However, in overdose situations, there may be an increase in the incidence of spontaneous abortion and fetal demise, especially when antidote treatment with N-acetylcysteine is delayed.13,16 Early studies linked aspirin to an increased risk of perinatal and neonatal bleeding, increased risk of postmaturity, prolonged labor, low birth weight, neonatal hypoglycemia, neonatal metabolic acidosis, and neonatal death.13,14 However, a number of meta-analyses in humans failed to demonstrate a teratogenic effect of aspirin,13,14,17,18 and in the Perinatal Antiplatelet Review of International Studies (PARIS) Collaboration, low doses of aspirin were actually found to be beneficial and to reduce the risk of preeclampsia, premature birth, and adverse perinatal outcomes.19 There was a trend, however, toward a slightly increased incidence of gastroschisis (a defect in the abdominal wall through which abdominal contents protrude) with use of nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin in the first trimester.13,18–20 Aspirin is excreted into breast milk, and because of its association with Reye’s syndrome, its use is therefore discouraged in breast-feeding.11–13 Prostaglandin synthesis inhibitors such as NSAIDs have been shown to block blastocyst implantation and may therefore inhibit conception.20 Their use in the first trimester has been linked to an increased risk of spontaneous abortions and a slight increase in cardiac septal defects, oral clefts, and gastroschisis.13,14,20–22 When they are used in the third trimester, NSAIDs inhibit labor and have been used as tocolytic agents for premature labor.13,14,21 When they are used in the latter part of pregnancy, NSAIDs have been linked to a number of negative effects on the neonate, most notably premature closure of the ductus arteriosus, leading to neonatal pulmonary hypertension and death.13,14,21,23 An increased incidence of fetal periventricular hemorrhages, fetal nephrotoxicity, oligohydramnios, and neonatal gastrointestinal hemorrhage has also been reported.13,14,21,23 Their use in the latter part of pregnancy is therefore discouraged. NSAIDs in general appear to be safe during lactation.12,13 In general, short-term, episodic use of opiates such as oxycodone, hydrocodone, morphine, and fentanyl appears to be safe in pregnancy.13,14 Their use near term, however, may result in severe respiratory depression of the neonate.13,14 In addition, prescribing of narcotics for long periods can lead to fetal addiction, low birth weight, and neonatal abstinence syndrome.13,14,24 Neonatal abstinence syndrome is characterized by CNS hyperirritability and autonomic nervous system dysfunction and a higher mortality in the offspring.24 The short-term use of opiates during lactation appears to be safe, but nursing infants should be closely monitored for respiratory depression.12,13 A case report of the death of a nursing infant whose mother was taking codeine for pain13,25 and a review of complications of codeine use in nursing mothers prompted the FDA to issue an advisory for codeine use in lactation. General anesthetics, sedative-hypnotics, and neuromuscular blockers are indicated for rapid sequence intubation (RSI). Some anesthetics and sedative-hypnotics are also used for procedural sedation. Most of the data regarding the use of these agents during pregnancy have been obtained from animal studies and from retrospective human data. None of the agents has been consistently associated with congenital malformations.26–28 Etomidate.: Etomidate is an ultra-short-acting hypnotic agent that is commonly used for procedural sedation or RSI. It has not been linked to congenital defects in animals even when high doses were used.13,14,26,27 Similarly, it is not associated with an increase in congenital anomalies in humans.13,14,26,27 However, newborns of mothers undergoing cesarean section with etomidate were found to have significant reductions in serum cortisol concentrations 1 hour after delivery.13,14,27,28 The significance of this effect remains unclear. Because etomidate is excreted in milk in very small amounts and decreases rapidly, it is most likely compatible with breast-feeding.13,14,28 Ketamine.: Ketamine is a rapidly acting dissociative anesthetic that is commonly used in pediatric procedural sedation and may be used in RSI. It is frequently used in obstetrics and has not been associated with fetal developmental malformations.13,14,26,27 Ketamine has a dose-dependent oxytocic effect and in high doses (>2 mg/kg) has been associated with uterine tetany.13,14,26,27 It may also result in increased maternal blood pressure and heart rate and an increased neonatal muscle tone.13,14 Neonatal depression has also been reported.13,14 Whereas there are no published data on the effects of maternal ketamine use on the nursing infant, the amount of ketamine in the mother’s plasma should be undetectable after 12 hours, and the American Academy of Pediatrics (AAP) considers it compatible with breast-feeding when it is used after this time.12–14 Midazolam.: Midazolam is a short-acting benzodiazepine commonly used in procedural sedation or RSI. It has not been linked to congenital defects in most laboratory animals even when high doses are used.13,14,26–28 In humans, there are no data available on its use in the first and second trimesters of pregnancy, but benzodiazepines do not appear to result in malformations in human observational studies.13,14,26–28 When it is used at term, midazolam has been associated with neonatal respiratory depression and decreased muscle tone.13,14,26–28 The AAP considers midazolam use during lactation of concern because there are few data addressing the issue.12 However, in a number of studies, the concentration of midazolam in breast milk was found to be negligible 8 hours after maternal ingestion.13,14,29 Propofol.: Propofol is a rapidly acting sedative anesthetic that is commonly used in RSI and procedural sedation. No data are available regarding its use in the first and second trimesters of pregnancy in humans, but it does not result in malformations in animal studies.13,14,26,27 Its use at term also appears to be safe, although high doses have been linked to neonatal respiratory and CNS depression.13,14,26–28 Propofol is thought to be compatible with breast-feeding because only negligible amounts are found in breast milk 24 hours after its use.13,14,26–29 Thiopental.: Thiopental is an ultra-short-acting barbiturate that may be used during RSI or for status epilepticus. It has not been linked to congenital defects in laboratory animals even when high doses are used.13,14,26–28 Similarly, it has not been associated with an increase in congenital anomalies in humans.13,14,26–28 Thiopental is excreted in milk in concentrations that are too low to be pharmacologically significant and is considered compatible with breast-feeding.12–14 Nondepolarizing Neuromuscular Blocking Agents.: Nondepolarizing neuromuscular blocking agents, such as rocuronium and vecuronium, are used in RSI. The effects of these agents on organogenesis in humans are not known, but these agents are not thought to pose a significant teratogenic risk because very little of the maternal dose crosses the placenta.13,14,26–28 Furthermore, according to the manufacturers, there was no increase in congenital anomalies noted when small laboratory animals were injected with rocuronium daily.13,14,26–28 Because of their chemical properties, very little of either drug is excreted in milk.13,14,26–28 Women are not expected to breast-feed while taking neuromuscular blockers. Depolarizing Neuromuscular Blocking Agents.: Succinylcholine is a depolarizing neuromuscular blocking agent used in RSI for its rapid onset of action and short duration of paralysis. It has not been associated with congenital defects, although there is limited experience with its use in early pregnancy in humans.13,14,26–28 In addition, it does not appear to have any effects on the newborn, except in rare cases of neonates with pseudocholinesterase deficiency.13,14,26–28 As occurs in adults with the same condition, newborns with cholinesterase deficiency exhibit prolonged respiratory depression and paralysis.13,14,26–28 Succinylcholine in lactation has not been studied; however, it is probably safe because it is hydrolyzed quickly.13,14 Benzodiazepines.: Benzodiazepines are commonly used for the sedation of the acutely agitated patient, in the treatment of acute seizures and alcohol withdrawal, and as a sedative during RSI. The short-term use of benzodiazepines during pregnancy appears to be safe. Case reports have linked their use during the first trimester of pregnancy to increased risk of oral clefts,10,13,14 but in an observational study based on the Swedish medical birth registry, no such association was found.13,14,30 Neonates exposed to benzodiazepines may exhibit signs of toxicity, including apnea, cyanosis, unresponsiveness, hypotonia, poor feeding, and withdrawal symptoms characterized by irritability and tremulousness.10,13,14 Because of the reported risk of apnea, it is recommended that neonates exposed to benzodiazepines through breast-feeding be monitored closely.10,12–14 Warfarin (Coumadin) is a known human teratogen and affects 4 to 5% of exposed fetuses. The risk from exposure is greatest during 6 to 9 weeks of gestation and seems to be dose dependent.14,15,31 The fetal warfarin syndrome is associated with multiple abnormalities, such as hypoplasia of the nasal bones, midline dysplasia including agenesis of the corpus callosum, optic atrophy and blindness, mental retardation, seizures, and stippling of the bones with scoliosis and shortening of limbs.10,14,15,31,32 Because warfarin is so highly protein bound, only a little is secreted into milk, and use by breast-feeding mothers is acceptable.12–14,31 Caution should be used in breast-feeding of premature infants because they may be at increased risk for intraventricular hemorrhage.13,31 Unfractionated heparin is a highly charged heterogeneous molecule with a molecular mass between 5 and 35 kDa.13 It does not cross the placenta and does not present a direct risk to the fetus.13,14 Early reports on the use of heparin for the prevention or treatment of venous thromboembolism during pregnancy noted an increased risk of prematurity, stillbirth, and fetal hemorrhage.14 However, these risks were recently attributed to the underlying maternal condition rather than to heparin.14,15,31,33 When anticoagulation during pregnancy is required, heparin is considered the agent of choice.13,14,33 Its use is sometimes associated with maternal osteopenia and immune-mediated thrombocytopenia and maternal hemorrhage at delivery.13,14,31,33 Patients need to be carefully monitored for these adverse effects. Because of its high molecular weight, heparin is not excreted in breast milk and is compatible with breast-feeding, but critically ill patients are not expected to breast-feed.12–14,31 Low-molecular-weight heparin may be used during pregnancy and in the postnatal period for therapeutic or prophylactic anticoagulation.14,15,31,33 All currently available low-molecular-weight heparin products have been used safely during pregnancy.14,15,31,33 Data are limited, however, because of their relatively recent introduction. Alteplase, reteplase, urokinase, and streptokinase have been used successfully in pregnant women in cases of life-threatening pulmonary embolus34 or myocardial infarction. Experience with these agents during pregnancy, however, remains limited. To date, no teratogenic effects have been reported in humans, but intrapartum maternal hemorrhage has been reported with alteplase and urokinase.14,15,31 Most thrombolytics are thought to be compatible with breast-feeding because of their short half-life.13,14 N-Acetylcysteine is a mucolytic agent that has been used successfully and without untoward effects in pregnant women who have overdosed on acetaminophen.13–16,35 No teratogenic effects have been reported, and pregnant patients who overdose on acetaminophen should be treated in the same manner as nonpregnant patients are.13–16,35 Although there are no reports of N-acetylcysteine use during lactation, it is most likely safe because it has been used in neonates without untoward effects.13,14,35 Deferoxamine is indicated for iron toxicity occurring from iron overdose or from multiple transfusions in thalassemia patients. It has been associated with developmental effects on ossification in some animal species.13,14,35,36 Experience in humans is limited, but it does not appear to affect the fetus.13,14,35,36 The effects of deferoxamine on the nursing infant are not known, but it is probably compatible.13,14 Digoxin immune fragment (Fab) therapy is indicated for life-threatening digitalis overdose and is being studied for treatment of preeclampsia.13,14,35 There are very few case reports of the use of digoxin immune Fab during pregnancy, and a conclusion on its effects on the offspring cannot be made.13,14 Thus in cases of life-threatening digitalis overdose with arrhythmias, the benefits of treatment of the mother outweigh the risk to the fetus. Digoxin immune Fab is not likely to be excreted in large amounts in milk, and it is probably safe for use during lactation.12–14 Dimercaprol or British antilewisite is a chelating agent that is used as an antidote for acute mercury, lead, arsenic, and gold poisoning.13,14,35,37 It has also been used in Wilson’s disease.13,14 It is teratogenic in mice and has been associated with increased mortality, growth restriction, cleft facial features, cerebral herniation, and abnormal digits, but experience in humans is limited.13,14,35,37 In general, in cases of heavy metal poisoning, the maternal benefits of treatment will outweigh the potential risks to the unborn fetus. Breast-feeding is contraindicated in patients poisoned by heavy metals.13,14 Flumazenil is a benzodiazepine antagonist. No teratogenic effects have been reported in animals, and data on humans are limited.13,14,35 Its use in pregnancy and lactation depends on the potential maternal benefit compared with possible risks to the fetus and nursing infant. Because it has a short half-life, breast-feeding may resume after a few hours.13,14 Fomepizole is a competitive inhibitor of alcohol dehydrogenase indicated in cases of methanol and ethylene glycol poisoning. Its use during pregnancy has not been studied in animals or humans. Its safety during pregnancy is not known.13,14,35 In cases of toxic alcohol poisoning, the benefits of treatment of the mother outweigh the possible risks to the fetus or nursing infant. Use of ethyl alcohol in these situations may be considered.13,14 Breast-feeding is not expected to continue during acute toxic alcohol poisoning. Hydroxycobalamin is a vitamin B12 derivative that is indicated in the treatment of cyanide toxicity. The effects of hydroxycobalamin on human pregnancy have not been studied, but benefits of its use in cyanide poisoning outweigh any risk to the fetus. Studies in animals do not reveal an association with any developmental abnormality.13,14 Breast-feeding is not expected to continue during cyanide poisoning, but hydroxycobalamin, like its related compounds, is considered compatible with breast-feeding.12–14 Methylene blue is used in the treatment of methemoglobinemia. In the past, it was injected into the amniotic sac to identify twins and to detect rupture of the membranes, but these practices were associated with hemolytic disease in the newborn, hyperbilirubinemia, and deep blue staining of the newborn.13,14,35,38 Methylene blue in pregnancy has also been associated with an increased incidence of intestinal obstruction and atresia in the newborn.13,14,35,38 Breast-feeding is not expected to continue during acute severe methemoglobinemia, but the effects of methylene blue on the nursing infant are expected to be minimal.13,14 Naloxone, used to reverse severe respiratory depression in opiate overdose, readily crosses the placenta. Although it has not been associated with reproductive abnormalities,13,14,35 its use during pregnancy results in increased fetal wakefulness, increased fetal movement, and increased heart rate, effects attributable to antagonism of fetal endorphins.13,14,35 In addition, its use in opiate-addicted mothers may precipitate withdrawal in both mother and term fetus.13,14,39 It is compatible with breast-feeding.12–14 Physostigmine is an anticholinesterase agent indicated in cases of severe anticholinergic poisoning associated with delirium. Experience with the medication during pregnancy is limited, and its effects on the developing fetus are unknown.13,14,35 Use of physostigmine at term was associated with only mild decreases of Apgar scores at 1 and 5 minutes.13,14,40 Breast-feeding is not expected to continue during anticholinergic poisoning, but physostigmine is thought to be probably compatible with breast-feeding.13,14 Pralidoxime is indicated for organophosphate/cholinergic poisoning because it is able to reactivate cholinesterase. Experience with pralidoxime in pregnancy is limited, and its effects on fetal development are not known.13,14,35,40 In cases of organophosphate poisoning, the benefits to the mother generally outweigh the possible risk to the fetus. Breast-feeding is not expected to continue during cholinergic poisoning. Pyridoxine is a vitamin required for good maternal health and good fetal development. It is indicated in isoniazid poisoning and in gyromitrin mushroom poisoning. Its use has been advocated by some for nausea and vomiting of pregnancy, gestational hypertension, and diabetes.13,14 It has not been associated with any adverse developmental effects, and it is safe in lactation.13,14 Succimer is a heavy metal chelator that is indicated in lead poisoning. It has also been used in arsenic, mercury, and cadmium poisoning. It has been linked to congenital defects in animal models, possibly because of its negative effects on zinc and copper metabolism.13,14,35 Experience with the use of succimer in human pregnancy is limited to case reports, which did not shed any light on its teratogenicity in humans.13,14,35,37,41 There are no reports of succimer use during lactation, but breast-feeding is contraindicated in heavy metal poisoning.13,14 Infections in the pregnant woman have the potential to adversely affect pregnancy outcome as well as fetal development. When they occur in the first trimester, they are a common cause of spontaneous abortion, and when they occur in the second or third trimester, they are the most common cause of preterm labor and delivery. Antimicrobial agents also may adversely affect the pregnancy. Aminoglycosides, for example, may be nephrotoxic and ototoxic in both the mother and her offspring,13,14,41,42 tetracyclines may result in dental staining of the developing fetus,13,14,41,42 and lincosamides may be skeletotoxic.13,14 The penicillins, cephalosporins, and macrolide antibiotics remain the drugs of choice for infections during pregnancy.41,42 Other classes of antibiotics are prescribed only if these have failed to control the infection or in cases of severe maternal intolerance to these drugs.13,14,41,42 The choice of antimicrobial therapy will depend on the age of the pregnancy, severity of infection, and maternal tolerance for the drug used. Many drugs also are secreted into breast milk. Potential problems for the neonate include direct effects on the neonate, changes in bowel flora, diarrhea, and potential interference with culture results.12–14 Aminoglycosides.: The association of aminoglycosides such as gentamicin, streptomycin, tobramycin, and neomycin with nephrotoxicity and ototoxicity is well known in the literature and in practice.13,14,41,42 Aminoglycosides, however, do not appear to have any structural teratogenic effect in humans,43 but kanamycin and streptomycin have been reported to cause ototoxicity in the mother and her offspring.13,14 There are no reports definitively linking in utero exposure to gentamicin, streptomycin, tobramycin, and neomycin to either ototoxicity or nephrotoxicity.13,14 Aminoglycosides are secreted in small amounts in breast milk and are poorly absorbed from the gastrointestinal tract.12–14 They are probably compatible with breast-feeding.12–14
Drug Therapy in Pregnancy
Perspective
Classification of Teratogenic Risk
Principles of Disease
Drug Transfer across the Placenta
Drug Transfer during Lactation
Drug Therapy during Pregnancy
Analgesic Agents
Aspirin
Nonsteroidal Anti-Inflammatory Drugs
Opiate Analgesics
Anesthetics, Sedative-Hypnotics, and Neuromuscular Blockers
Anesthetics
Neuromuscular Blockers
Sedative-Hypnotics
Anticoagulants and Thrombolytics
Heparin
Thrombolytic Agents
Antidotes
Deferoxamine
Digoxin Immune Fragment
Dimercaprol
Flumazenil
Fomepizole
Hydroxycobalamin
Methylene Blue
Naloxone
Physostigmine
Pralidoxime
Pyridoxine
Succimer
Antimicrobial Agents
Antibiotics
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Drug Therapy in Pregnancy
Only gold members can continue reading. Log In or Register to continue