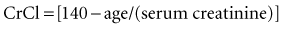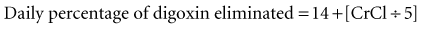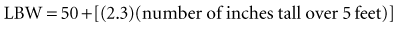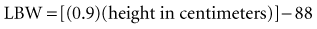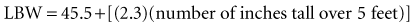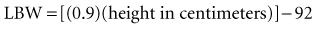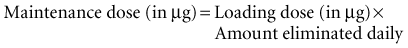177 Digitalis
 Therapeutic Indications
Therapeutic Indications
Digoxin is indicated for the treatment of mild to moderate congestive heart failure (CHF) and for the control of ventricular response rates in patients with chronic atrial fibrillation.1 Digoxin improves left ventricular ejection fraction, improves exercise tolerance, ameliorates CHF-related symptoms, and decreases CHF-related hospitalizations and emergency care. But treatment with digoxin has not been shown to improve survival in patients with systolic left ventricular dysfunction.2–4 However, digoxin is not indicated as primary treatment for stabilization of acutely decompensated heart failure.5
In the critical care setting, digoxin may be used to treat atrial arrhythmias, predominantly atrial fibrillation.6 In chronic atrial fibrillation, digoxin is useful for controlling the ventricular rate in patients with left ventricular systolic dysfunction.5 Rate control occurs in a linear dose-response fashion over a range of digoxin doses from 0.25 to 0.75 mg/d for adults,1 but the drug may not consistently control ventricular rate in dysfunctional states associated with increased sympathetic tone, such as exercise- or emotional stress–induced tachycardia.6–9 In acute atrial fibrillation, digoxin provides effective ventricular rate control and represents a useful therapy for rate control, especially if left ventricular function is compromised.1,10 The agent does not restore normal sinus rhythm, although occasionally atrial fibrillation spontaneously resolves during initial therapy.5
 Mechanism of Action
Mechanism of Action
Digoxin is a cardiac glycoside with specific effects on the myocardium. Inhibition of the sodium/potassium–adenosine triphosphatase (Na+/K+-ATPase) pump increases intracellular sodium concentration and subsequently increases intracellular calcium concentration by stimulation of sodium-calcium exchange.1,11 The pharmacologic effects of digoxin include increased force of systolic contraction (i.e., positive inotropic activity); decreased activation of the sympathetic nervous system and renin-angiotensin system (neurohormonal deactivating effect); sensitization of arterial baroreceptor nerve endings, which then normalizes the reflex vasodilation response to cardiac unloading; and decreased heart rate and conduction velocity within the atrioventricular (AV) node (vagomimetic effect). Neurohormonal effects occur at low dosages, independent of inotropic effects. Hemodynamic improvement is observed in CHF related to both the inotropic and neurohormonal effects of digoxin. The vagal effects of digoxin result in slowed conduction and prolongation of AV node refractoriness, which slows the ventricular response in patients with atrial fibrillation. The overall response to digoxin is an increase in cardiac output and reduction in pulmonary artery pressure, systemic vascular resistance, plasma norepinephrine level, and pulmonary capillary wedge pressure. Minimal changes in blood pressure occur with initiation of therapy.1,12–13
 Pharmacokinetics
Pharmacokinetics
Intravenous (IV) preparations are 100% bioavailable, whereas most oral formulations provide only 60% to 80% bioavailability.1 Capsules containing liquid have increased bioavailability, being about 90% to 100% of the IV formulation. Therefore, dosing considerations are important when switching between oral and IV preparations. Digoxin absorption occurs primarily in the small intestine. When some digoxin oral preparations are taken after meals, the rate of absorption is slowed, but the total amount of digoxin absorbed remains unchanged.1 Impaired absorption after oral administration can occur if intestinal function is impaired, although partial gastrectomy or jejunoileal bypass does not affect absorption to an appreciable extent.1,14–15
The distribution phase of digoxin metabolism is prolonged after oral or IV administration. For patients started on oral therapy, the onset of action occurs within 0.5 to 2 hours, and peak effects are seen within 6 to 8 hours.1 After IV administration, onset occurs in 5 to 30 minutes, and peak effect is observed within 1 to 5 hours.16 This delay in pharmacologic effect may be undesirable in the setting of acute atrial fibrillation. Pharmacologic effects typically persist for 3 to 4 days after withdrawal of digoxin therapy.
Approximately 20% to 30% of digoxin is protein bound in patients with normal renal function or uremia.1 Digoxin is extensively bound to multiple tissues, particularly to Na+/K+-ATPase in cardiac and skeletal muscle, and demonstrates a large volume of distribution, which averages 6 to 7 L/kg of total body weight in patients with normal renal function. A decrease in the volume of distribution occurs in patients with renal dysfunction or dialysis.
With normal renal function, the elimination half-life is 36 to 48 hours. Elimination is prolonged in patients with renal dysfunction, being about 3.5 to 5 days in anuric patients.11 Metabolism occurs primarily in the liver, but the drug also is metabolized by bacteria within the large intestine after oral administration.1 Excretion of digoxin is predominantly in the urine as unchanged drug. The drug is cleared by glomerular filtration and active tubular secretion. Small amounts are excreted in bile and feces. Approximately 30% of the total digoxin load in the body is eliminated daily in patients with normal renal function. The metabolism and excretion of digoxin is not appreciably altered in patients with liver disease if normal renal function is present. Importantly, increased urinary output does not result in enhanced elimination of digoxin, because elimination is dependent on age, gender, and serum creatinine. Estimations of creatinine clearance (CrCl) in milliliters per minute can be calculated from the patient’s age (in years) and the serum creatinine concentration (in mg/dL) by the modified Cockcroft and Gault equation:
 Dosing Recommendations
Dosing Recommendations
General Considerations
Lean body mass should be used to calculate the appropriate digoxin dosage for adult patients in intensive care units (ICUs), because no appreciable amount of digoxin is distributed to body fat.17 Age, renal function, and weight all have to be considered when calculating both loading and maintenance doses for initiation of digoxin therapy.18 Digoxin dosages in the pediatric population must be carefully titrated, especially in neonates. For children from infancy to age 10 years, substantially higher dosing is necessary in comparison with adult patients (see later discussion). In addition, concomitant medications (discussed later) may influence serum digoxin levels and should be considered when initiating therapy.
Initial Loading Dose
Recent literature does not support initial bolus dosing for patients with CHF.18 If deemed appropriate, a daily dose of 8 to 12 µg/kg is suggested for adult patients in heart failure who are in normal sinus rhythm. Adult patients with CHF may receive initial dosing (62.5 to 250 mg/day), based on ideal body weight and kidney function.19 In the acute setting, administering an initial loading dose is recommended for management of supraventricular tachyarrhythmias. Determining lean body weight (LBW in kilograms) is necessary for calculating digoxin loading and maintenance dosing. Appropriate dosing weight can be calculated from the following equations:
or
or
An initial IV loading dose for adults of 10 to 15 µg/kg based on LBW is necessary for adequate ventricular rate control in the setting of atrial fibrillation or atrial flutter. Patients with impaired renal function and those older than 70 years of age require lower initial loading doses; a 50% dose reduction is recommended. Typically, the loading dose is administered as approximately half of the total dose immediately (maximum of 500 µg administration at one time), followed in 6 to 8 hours by 25% of the total dose, with the remaining 25% given after another 6 to 8 hours.18 For example, a loading dose of 1000 µg should be administered as a 500-µg IV bolus, followed by 250 µg IV every 6 hours for 2 doses. To prevent toxicity, a thorough clinical evaluation of the ICU patient should be completed before additional bolus doses are given during the loading dose phase of therapy.
Maintenance Dosing
If the initial loading dose of digoxin successfully controls the ventricular response of a supraventricular arrhythmia, a maintenance dose should be initiated.18 The maintenance dose is also determined by renal function and the patient’s LBW. The maintenance dose needed by patients not previously receiving digoxin therapy can be estimated from the loading dose and the percentage of drug eliminated each day as follows:
Patients who are switched from IV to oral therapy must have dosage adjustments made as necessary.18 If changing from IV therapy to oral tablets or elixir, the digoxin dosage should be increased by approximately 20% to 25%. However, no dosage adjustment is needed if the oral therapy uses liquid-filled capsules. For example, 100 µg of the IV product is approximately equivalent to 100 µg of the liquid-filled capsules (Lanoxicaps) or 125 µg of the tablet (Digitek, Lanoxin) or the elixir formulation.
 Special Populations
Special Populations
Thyroid Dysfunction
Thyroid dysfunction results in an altered pharmacodynamic profile. Hypothyroid patients require decreased digoxin dosages compared to euthyroid ICU patients.14,15,18 Hyperthyroid patients commonly need increased digoxin dosages, potentially secondary to increased resistance to digoxin therapy. Alterations in absorption, tissue distribution, renal excretion, and sensitivity of digitalis receptors in patients with thyroid disease have been proposed as mechanisms to explain altered serum digoxin concentrations.14,15
Electrolyte Disturbances
Hypokalemia enhances the effects of digoxin by increasing the cardiac effects due to depletion of intracellular potassium.18 Hypomagnesemia requires larger digoxin doses for rate control in the setting of atrial fibrillation.18 Repletion of potassium and magnesium to adequate levels should be completed before initiation of digoxin therapy to prevent potential proarrhythmic effects. Significant hypercalcemia may enhance digoxin toxicity.18
Heart Disease
For patients with coronary artery disease, cor pulmonale, or extensive myocardial damage including previous myocardial infarction, a reduction of digoxin dosage may be necessary.18 Digoxin has been reported to increase mortality in patients with acute ischemic syndromes,20,21 although more recent data do not support this idea.16 The increase in sensitivity to digoxin based on underlying cardiac disease mandates caution and careful patient monitoring.
Gender
When used to treat heart failure and decreased left ventricular function, digoxin was found to have different effects on all-cause mortality in men compared to women.22 Specifically, digoxin was associated with increased all-cause mortality among women in a population with heart failure and depressed left ventricular systolic function.22 The impact of gender on the pharmacologic effects of digoxin used to treat supraventricular arrhythmias is currently unknown, and dosage adjustments are not recommended on the basis of gender at this time.
Pregnancy
Digoxin is a category C medication and should be considered for pregnant patients only if the benefits clearly outweigh the risks, and no alternative is available. The impact in terms of fetal harm or reproductive capacity is unknown.1
Pediatrics
Individualized dosing is extremely important in pediatric patients. In newborns, a reduction in renal clearance of digoxin is observed, necessitating dosage adjustments, especially in premature infants.18 Divided daily dosing is often necessary in infants and those younger than 10 years of age. The elixir formulation is especially suitable for the pediatric population. Loading dosages of the pediatric elixir differ based on age: 20 to 30 µg/kg for premature infants, 25 to 35 µg/kg for full-term newborns, and 35 to 60 µg/kg for children younger than 2 years of age. For children aged 2 to 5 years, oral loading doses of 30 to 40 µg/kg are appropriate, and for those aged 5 to 10 years, the oral loading dose is 20 to 35 µg/kg. Children older than 10 years of age require 10 to 15 µg/kg initially. Maintenance doses for pediatric patients are approximately 25% of the oral loading dose necessary to achieve the optimal therapeutic effect. If IV therapy is necessary, the dose is approximately 80% of the total oral elixir requirement.
 Therapeutic Monitoring
Therapeutic Monitoring
Measurements of digoxin concentration are useful in certain situations to assist in evaluating the effects of the drug on the disease state being treated and to avoid toxicity.18 For treatment of supraventricular tachyarrhythmias, the usual therapeutic range for serum digoxin concentration is 1 to 2 ng/mL. However, patients can require serum concentrations as great as 3 ng/mL. The concentration is correlated with effectiveness or toxicity in a particular patient. The same level that is toxic in one patient may be therapeutic in another. Therefore, dose titration should be based on the heart rate and signs or symptoms of toxicity rather than the absolute digoxin concentration.
Evidence to support the use of serum concentrations to ensure efficacy in the treatment of heart failure is lacking. Lower digoxin concentrations (0.5-0.8 ng/mL) appear to provide equal or superior efficacy and avoid toxicity. Gheorghiade et al.23 found that exercise time, heart failure scores, heart rate, and neurohormonal findings were similar among patients with serum digoxin concentrations of 0.67 ± 0.22 ng/mL compared to those at 1.22 ± 0.35 ng/mL. Mean concentrations of 0.8 ng/mL provided a reduction in rate of hospitalizations and worsening heart failure.4,24 Rathore et al.25 demonstrated that patients with digoxin concentrations of 0.5 to 0.8 ng/mL had a reduction in absolute mortality rate of 6.3% compared with patients who received placebo. However, no reduction in mortality was observed for patients with concentrations of 0.9 to 1.1 ng/mL compared to the placebo group, and an increase in mortality was found for patients with levels of 1.2 ng/mL or greater.
Measurements of serum digoxin concentrations may be particularly useful when kinetic parameters are changing.18 For example, in patients with improving or declining renal function or in situations in which a drug interaction could decrease absorption or digoxin clearance, monitoring levels is helpful. Digoxin concentrations can be obtained periodically to detect excessive drug levels and prevent toxicity.
Proper timing of digoxin measurements is critical. Although digoxin is found in the plasma compartment within a brief period after administration, the medication distributes slowly into the heart and other tissues.26 Because the heart is the site of action, digoxin concentrations measured less than 4 hours after IV administration or 6 hours after oral administration are misleading. The optimal time to measure digoxin levels is 12 to 24 hours after administration. For patients with normal renal function, digoxin concentrations do not reach steady state for 7 to 10 days in the absence of a loading dose. As renal function declines, clearance of digoxin is impaired, and the time to reach steady state is prolonged. In patients with end-stage renal failure, this duration is extended to 15 to 20 days. Levels obtained before the drug has reached steady state can be useful to prevent toxicity or assess a trend. However, these concentrations do not reflect the maximum concentration at steady state.