CHAPTER 35 DIAGNOSTIC AND THERAPEUTIC ROLES OF BRONCHOSCOPY AND VIDEO-ASSISTED THORACOSCOPY IN THE MANAGEMENT OF THORACIC TRAUMA
Direct injury to the chest and pulmonary complications after any major trauma account for a significant proportion of trauma-related morbidity and mortality. In the past the role of thoracic endoscopy was limited to bronchoscopic diagnosis of major airway injury and assistance with pulmonary toilet. Major injuries to the tracheobronchial tree, significant hemorrhage within the chest, failure of nonoperative management of chest injuries, and major pulmonary complications invariably required thoracotomy with its high morbidity and mortality. Technical advances in fiber optics and videoscopic imaging have led to rapid advances in the field of minimally invasive surgery. In the thoracic region, this has led to the advent of videoassisted thoracoscopic surgery (VATS) and the broadening of the role of bronchoscopy, both in terms of diagnosis and therapy. These minimally invasive endoscopic techniques have significantly lower morbidity and mortality. The current chapter focuses on the evolving role of thoracic endoscopy (thoracoscopy and bronchoscopy) following chest trauma and major pulmonary complications after any trauma.
INCIDENCE
Thoracic trauma accounts for a significant burden of disease in terms of morbidity and mortality. Twenty percent of trauma-associated deaths involve chest injury, and chest trauma is second only to head and spinal cord injuries as a cause of death following trauma.1 Death as a result of chest trauma in the acute setting is related to either airway injury, direct trauma to the heart, or massive bleeding in the chest cavity. In addition to the acute deaths that are a direct result of chest trauma, pulmonary complications following chest trauma or any trauma add to the mortality and morbidity attributable to the chest.2
Most chest trauma comprises of abrasions, rib fractures, and simple pneumothoraces that are easily diagnosed with chest x-rays and/or computed tomography (CT) and can be treated with simple measures—pulmonary toilet, pain management, and tube thoracostomy. The indications for open thoracotomy in the acute setting are principally major airway disruption and massive bleeding in the chest or airway. Approximately 1% of all trauma admissions require open thoracotomy in the acute setting.3 Open thoracotomy in this setting is associated with a very high morbidity and mortality.3 Bronchoscopic control of bleeding within the airway and stenting of major airway injury can offer a lower-risk alternative to open surgery in selected patients.4,5 Similarly, VATS offers a lower-risk alternative for managing hemorrhage within the chest cavity.6 In the nonacute setting, trauma patients with or without direct injury to the chest have a high incidence of pulmonary complications, including pneumonia, retained hemothorax, fibrothorax, empyema, and acute respiratory distress syndrome (ARDS), posing diagnostic and therapeutic challenges.1,2 Thoracic endoscopy in this setting is playing an increasingly important role in early diagnosis and therapy for these complications, and possibly improving outcome.
DIAGNOSTIC AND THERAPEUTIC ROLES OF VATS IN CHEST TRAUMA
Indications and Patient Selection
The first diagnostic and therapeutic application of thoracoscopy for the management of thoracic pathologies such as pleural effusions, pleural adhesions, empyemas, and thoracic malignancy, was recorded by Jacobeaus at the University of Stockholm in 1922.7 Thoracoscopy for the treatment of traumatic injuries was first described in 1946 by Branco for the management of hemothorax in penetrating chest injuries.8 In the last decade, the advent of minimally invasive access to the thoracic cavity combined with video-assisted technology and selective lung ventilation has revolutionized the diagnosis and the treatment of thoracic injuries with improved use and outcomes.9,10
Patient selection is very important for the application of VATS in thoracoabdominal trauma. The current indications are both diagnostic and therapeutic and include mainly the evaluation of a structural injury (the diaphragm, the pericardium, lung parenchyma, the thoracic duct, etc.) or the drainage of a pleural collection and repair of any structural damage. Aside from the usual bleeding disorders, the main contraindications of VATS include an unstable patient, or a patient with underlying lung and cardiac pathologies that preclude the use of single lung ventilation. Table 1 lists the current indications and contraindications for the use of VATS.
Table 1 Indications and Contraindications of VATS in Trauma
| Indications | Contraindications |
|---|---|
| Persistent pneumothorax | Hemodynamic instability |
Poor lung and cardiac functions with inability to tolerate single lung ventilation (chronic obstructive pulmonary disease, heart failure) | |
| Empyema | Massive hemothorax (>1.5 liters initially or 200 ml/hr over 3–4 hours) |
| Detection of intrathoracic organ injury (diaphragm, lung, thoracic duct, pericardium) | Obliterated pleural cavity (infection, pleuritis, previous surgery) |
| Intrathoracic foreign body | Suspected cardiac injury |
| Acute hemorrhage in stable patients | Indication for laparotomy |
Diaphragmatic Injuries
The incidence of blunt diaphragmatic injuries have been reported as low as 0.8% and as high as 7%.11,12 Blunt trauma accounts for 10%–30% of traumatic diaphragmatic ruptures (TDR) in North American series from urban trauma centers.13 The incidence of diaphragmatic injuries has been reported to be as high as 67% in penetrating thoracoabdominal trauma.14
Diaphragmatic injuries are particularly difficult to diagnose with the use of radiographic imaging such as chest x-ray or CT and can be missed in up to 30% of patients.14,15 Together with laparoscopy, thoracoscopy has become the diagnostic tool of choice for diaphragmatic injuries when compared with other nonoperative modalities.16,17 Thoracoscopy is particularly useful when laparoscopy may not be optimal or feasible. It is useful for evaluation of right-sided diaphragmatic injuries and of posterior wounds from the posterior axillary line to the spine.18 It is also useful for avoidance of abdominal procedures, particularly in patients with previous laparotomies and expected presence of extensive adhesions.
The therapeutic role of VATS in the treatment of diaphragmatic injuries is well documented.14,19 In a report of 24 patients who underwent VATS for thoracic injuries, 9 of 10 patients were successfully diagnosed with diaphragmatic injuries. VATS was used for repair of the diaphragm in four patients.19 Martinez et al.14 evaluated 52 patients with penetrating thoracoabdominal trauma admitted to the General Hospital for Accidents in Guatemala City. VATS was used to diagnose 35 patients with diaphragmatic injuries. All 35 diaphragmatic injuries were successfully repaired thoracoscopically. Even though successful thoracoscopic repair of diaphragm injuries is reported as feasible, safe, and expeditious, currently no long-term outcome results are available.
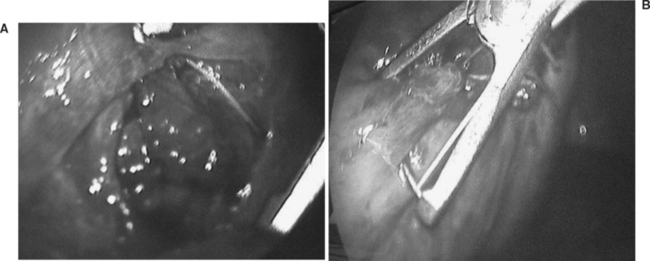
In areas where other abdominal injuries are suspected, a laparoscopic or open surgical approach is preferable depending on the available surgical expertise. A combined thoracic and abdominal cavitary endoscopy can also be useful. Figure 1 shows the thoracoscopic evaluation of a right diaphragm injury from an impaled object in the chest of a patient evaluated in our trauma center. This was followed by the thoracoscopic repair of the diaphragm after the laparoscopic confirmation of a nonbleeding liver laceration, and no other associated abdominal injuries. In all cases where a diaphragm injury is found, an exploratory laparoscopy or laparotomy is mandatory to rule out associated intra-abdominal injuries.
Retained Thoracic Collection
The evacuation of a retained hemothorax is one of the main indications for VATS. Inadequate evacuation of blood from the pleural space and prolonged thoracostomy tube drainage put the patient at risk for developing empyema and fibrothorax with prolonged hospital stays and increasing costs.9,20 The incidence of a retained hemothorax and empyema post–tube thoracostomy placement ranges from 4% to 20% and from 4% to 10%, respectively.8,9,19,21–23 A prospective randomized study of 39 patients with thoracic trauma and retained hemothoraces from Parkland Memorial Hospital showed that early evacuation with VATS compared with conventional therapy of a secondary chest tube placement lead to a significantly shorter duration of tube drainage (2.5 days), shorter hospital stay (2.7 days), and reduced hospital costs ($6000).9 These advantages of VATS were attributed to rapid and complete evacuation of the pleural space, optimal video-assisted positioning of the thoracostomy tubes, and identification and treatment of the sources of the bleeding and of other associated intrathoracic injuries.
It is important to note that these advantages rest on the early use (day 4–7 postinjury) of VATS for the evacuation of the hemothorax. In a study by Heniford et al.,20 19 of 25 patients (76%) with retained hemothorax were successfully treated with VATS. Failure of VATS correlated with time interval from injury to operation, and with the type of fluid collection (hemothorax vs. empyema). The mean time between admission and operation for the successful versus unsuccessful thoracoscopic drainage was 4.5 days and 14.5 days, respectively.20
The application of VATS for traumatic empyema is dependent on the phase of the empyema. For the acute/exudative empyema occurring between 1 and 5 days, VATS is uniformly effective. The success rate for the transitional/fibrinopurulent stage (day 6–14) is around 75%–85%, with a sharp drop to around 50% in the organized/chronic phase (>2 weeks).24,25 The presence of a thick fibrin peel with entrapped lung requires mature surgical judgment for early conversion to open thoracotomy and avoidance of the risk of further pulmonary parenchymal injury or the creation of a bronchopleural fistula.
Persistent Hemorrhage
Video-assisted thoracoscopic surgery is also useful for patients with persistent but slow hemorrhage and no hemodynamic instability. Unstable patients with suspected thoracic bleed require open thoracotomy. Smith et al.26 performed VATS on five hemodynamically stable patients for persistent hemorrhage from intercostal vessels. In three patients, the bleeding was successfully controlled with diathermy. Other techniques for hemorrhage control, including endoclips or argon beam coagulators, can be used.15 Intracorporeal suture placement around the rib was used successfully in our center for control of a persistent intercostal bleed not amenable to endoclip placement. The success rate for the thoracoscopic control of a nonhemodynamically compromising hemorrhage is around 80% with a thoracotomy conversion rate of 15%–20%.24,27
Persistent Pneumothorax
The incidence of persistent air leak and lung re-expansion 72 hours post–thoracostomy tube placement ranges from 4% to 23%.27,28 Conservative management with continuous pleural suction leads to prolonged chest tube drainage, prolonged hospital length of stay (LOS), and increased hospital costs.28 VATS has been shown to be safe and effective in the treatment of persistent pneumothorax with decreased number of chest tube days, hospital LOS, and cost.9,28,29 In our trauma center, endo-GIA staplers are routinely employed to staple off the affected lung parenchyma. Recently the use of a topical synthetic nonreactive surgical sealant (Coseal by Baxter, Freemont, CA) for the creation of an elastic watertight seal has also been reported.17 Chemical pleurodesis or pleural scarification by electrocoagulation remains a viable option especially in recurrences post-VATS. Carillo et al.28 reported the successful use of VATS in 10 of 11 patients with persistent pneumothorax post-traumatic injuries. The 11th patient was successfully treated with chemical pleurodesis. The inflammatory reaction that occurs with chemical pleurodesis, however, is often associated with increased pleural edema, drainage, and postoperative pain.15 Prior to committing a patient to VATS, it is important to aggressively evaluate the cause of the air leak and rule out a malfunctioning or a malpositioned chest tube, the presence of a foreign body, or a deeply penetrating rib fragment. The patient should be evaluated thoroughly with chest CT and bronchoscopy to evaluate the tracheobronchial tree, the distal parenchyma, and the pleural cavity.
Other Indications/Application
Other indications for use of VATS in chest trauma include diagnosis of bronchopleural fistulas, removal of retained foreign bodies, ligation of injured thoracic duct, drainage of chylothorax, and assessment of cardiac and mediastinal structure.10,15 Although pericardioscopy for suspected penetrating cardiac injury has been reported as feasible and safe in the hemodynamically stable patient, it remains very controversial, with potential for iatrogenic life-threatening injuries.30 In a stable patient, the gold standard approach for suspected cardiac injury remains the use of echocardiography or subxyphoid pericardial window followed by immediate sternotomy or left thoracotomy for evacuation of hemopericardium and repair of cardiac injury.
Morbidity and Complication Management
The reported complication rates for thoracoscopy are less than 10% and the missed injury rates are less than 1%.24,31 The perioperative complications include intrathoracic bleed (parietal, intercostal, or parenchymal), recurrent pneumothorax, and hemothorax. Other complications include intercostal neuritis and iatrogenic lung laceration. Conversion to open thoracotomy is reported to be less than 8% and usually results from pleural adhesions or uncontrollable bleed.31 This underscores the importance of the timing of the procedures, within 5–7 days—early enough to avoid pleural adhesions and fibrosis, and late enough to ensure adequate hemostasis. Persistent air leak in the postoperative period is attributed to underlying lung pathology such as emphysema or apical bleb disease. Late complications are rare and include the development of pneumonia, pleural edema, and empyema.9,31 Airway complications from malpositioned dual-lumen endotracheal tubes or the development of tension pneumothorax during one lung ventilation have also been reported.32
DIAGNOSTIC AND THERAPEUTIC ROLE OF BRONCHOSCOPY
Attempts to directly visualize the interior of the airway date as far back as the time of Hippocrates. However, the first recorded bronchoscopy was performed by Gustav Killian of Frieburg, Germany in 1887. The only available instrument at that time was a rigid bronchoscope and the principal indications were therapeutic, the commonest being removal of inhaled foreign objects. The field was advanced by Chevalier Jackson, the father of American bronchoesophagology, who designed modern rigid bronchoscopes. In 1963, Shigeto Ikeda introduced the flexible fibroptic bronchoscope, primarily as a diagnostic instrument.33 Flexible bronchoscopes were much easier to use, and flexible bronchoscopy became a diagnostic tool with wide application. The only therapeutic indication that persisted was removal of foreign bodies from within the tracheobronchial tree. Recent technical advances in the instrument itself and in the availability of other therapeutic tools such as stents, electrocautery, lasers, and so on, are allowing bronchoscopy to regain a role in therapy and also broadening its well established diagnostic role. While in some very limited situations rigid bronchoscopy may offer some advantages, the ease of use and greater experience in the use of flexible bronchoscopes have made rigid bronchoscopy rare in trauma settings.
Basic Technique of Flexible Fibroptic Bronchoscopy
Preparation
The patient should be placed on 100% oxygen, and the ventilator rate set at 10–12 breaths per minute. Adequate sedation is essential to avoid inducing stress. This is especially important in head injured patients as an acute rise in intracranial pressure (ICP) is well documented during bronchoscopy.34 We use a benzodiazepine (Ativan or Versed 2–4 mg intravenously) and a narcotic analgesic (morphine 4–8 mg intravenously). While bronchoscopy can be performed without paralysis, we have found that, to be able to perform it comfortably, temporary paralysis using vecuronium (10 mg intravenously) is very helpful. Adequate time should be given to allow the patient to get preoxygenated and the medications to take effect before starting the procedure. Recently, bispectral EEG monitoring (BIS) is being used to determine optimal sedation. Extra medications should be available to be given whenever needed to avoid stress to the body.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree