Fig. 66.1
Plain abdominal film
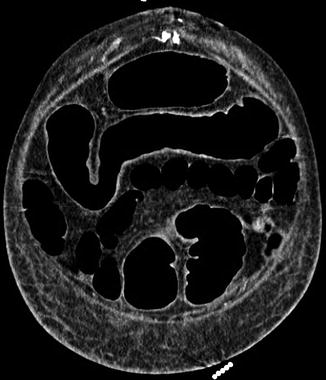
Fig. 66.2
Coronal view CT abdomen
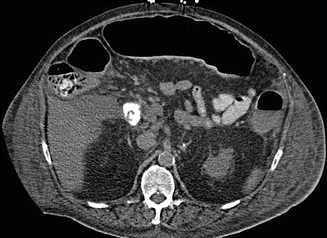
Fig. 66.3
Axial view CT abdomen
Principles of Management
Symptoms of C. Difficile Infection
Patients may present with a wide range of clinical manifestations, varying from asymptomatic carriage to shock and colon perforation. Asymptomatic carriage is more frequent than previously thought, ranging geographically from 4.4 to 23.2 % of patients admitted from the community [1]. Watery diarrhea, with or without the presence of mucus, is the classic presenting symptom, but patients may eventually progress to colitis, megacolon, and shock. Significant neutrophilic leukocytosis and acute kidney injury are commonly found with severe disease. Extracolonic manifestations, including bacteremia and ileitis, are rare, but when present are associated with a 25 % mortality rate [2].
Diagnosis
Diagnosis can be made by several methods, including toxin enzyme immunoassays, toxin gene nucleic acid amplification tests, direct culture, and detection of glutamate dehydrogenase. Toxigenic assay method is preferred, as some patients may be asymptomatic carriers [3]. Direct visualization of pseudomembranes can be performed, however the specificity is low for C. difficile and operator-dependent, and the procedure carries an increased risk of perforation [4].
Risk Factors
The predominant risk factor for acquisition of C. difficile infection is antibiotic use, particularly clindamycin, cephalosporins, and fluoroquinolones. Other risk factors include inflammatory bowel disease, immunodeficiency, malnutrition, solid organ transplantation, and hematopoietic stem cell transplantation. Use of proton pump inhibitors has conflicting evidence as a risk factor for C. difficile infection [5].
Severity of Disease
The Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA) have described criteria for defining severity of disease, based on presence of leukocytosis, AKI, and shock (Table 66.1) in 2010. Medical therapies are aimed at treating C. difficile colitis based upon the severity of disease [6].
Table 66.1
IDSA/SHEA 2010 C. difficile treatment recommendations
Clinical definition | Supportive clinical data | Recommended treatment | Strength of recommendation |
|---|---|---|---|
Initial episode, mild or moderate | WBC ≤15,000 and serum creatinine <1.5× baseline | Metronidazole 500 mg PO TID for 10–14 days | A-I |
Initial episode, severea | WBC ≥15,000 or serum creatinine ≥1.5× baseline | Vancomycin 125 mg PO QID for 10–14 days | B-I |
Initial episode, severe, complicated | Hypotension or shock, ileus, megacolon | Vancomycin 500 mg PO QID plus metronidazole 500 mg IV q8h. Consider PR vancomycin if ileus present | C-III |
First recurrence | Same as for initial episode | A-II | |
Second recurrence | Vancomycin in a tapered and/or pulsed regimen | B-III |
Medical Therapies
Clostridium difficile infection can induce hypovolemia, shock, and multiple electrolyte abnormalities; hence, a cornerstone of appropriate treatment is supportive critical care. Several antimicrobials are currently available to treat C. difficile infection. Metronidazole, a commonly used initial therapy for non-severe Clostridium difficile infection despite lack of FDA approval, is a nitroimidazole antibiotic which functions by interrupting the helical form of DNA. Vancomycin inhibits cell wall synthesis of C. difficile. It must be administered via an enteral route and it is the drug of choice for severe disease, and prolonged treatment regimens have been devised to successfully treat repeatedly recurrent disease. A staggered taper and withdrawal method of vancomycin administration with a probiotic has been described to be as effective as fecal microbiota transplant [7].
Fidaxomicin is a macrocyclic antibiotic that inhibits RNA polymerase in Gram-positive bacteria. It was approved by the FDA in April 2011 for treatment of C. difficile infection after being found to be non-inferior to treatment with vancomycin. As of now, a major limiting factor to fidaxomicin’s use is cost of therapy, though there is data that suggests it may reduce recurrence rates in select populations, which may offset the increased cost. Rifaximin is a less commonly used treatment adjunct that inhibits RNA polymerase, thereby interfering with transcription of DNA. Administration had previously been used anecdotally after a course of standard antibiotic therapy as a “chaser” with seemingly good results. A recent randomized, double-blinded study demonstrated that administration of rifaximin 400 mg PO TID for 20 days after completing standard C. difficile therapy resulted in fewer recurrences of infection [8].
Guidelines published in the American Journal of Gastroenterology provide similar recommendations regarding medical therapies guided by severity of disease [9].
Surgical Intervention
When medical therapies fail and patients develop worsening sepsis and shock, surgical intervention is warranted. There are multiple approaches to this including partial or total colectomy. Recently, more surgeons have been using the approach of creation of a diverting ileostomy with vancomycin colonic lavage. Studies have demonstrated reduced mortality and morbidity compared to historical controls when this method is pursued [10].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







