Chapter 21 Developing protocol and guidance to support assessment services
Paul Knight Liz Kenny
SUMMARY
This chapter will describe:
• definitions of guidelines and protocols
• approach to development of perioperative guidelines
• practical considerations in reviewing existing guidelines and protocols
• a case study from the UK NHS.
INTRODUCTION
Like it or not, guidelines and protocols are becoming an increasing part of modern life in general and healthcare in particular. But one must be aware right from the start of the difference between guidelines and protocols, a distinction not lost on politicians and lawyers in other walks of life. In the Scott Enquiry on export of defence equipment to Iraq, Lady Thatcher1 demonstrates an awareness of the real scope of guidelines when she states: ‘They are what they say, guidelines, they are not the law. They are guidelines.’
In this chapter we will discuss the difference between clinical guidelines and clinical protocols, how they work harmoniously in the healthcare arena, and the legal aspects of working with clinical protocols and clinical guidelines. We will show the reader how integrated care pathways (ICPs) use and promote the use of clinical guidelines and clinical protocols for the benefit for patients. The use of clinical protocols and guidelines is paramount in the preoperative assessment (POA) specialism if nurses are to be equipped to work in nurse-led clinics so we will be discussing the sort of guidance needed in POA and the considerations involved.
Finally, the authors will discuss how they and their team members managed the successful development of POA clinical protocols and clinical guidance in their hospital.
Clinical protocols and clinical guidelines:
What are they and why do we need them?
There are various dictionary definitions of the term ‘protocol’; however, definitions related to ‘clinical protocols’ are as follows:
• ‘the plan for a course of medical treatment or scientific experiment’2 or
• ‘a document that describes the objective(s), design, methodology, statistical considerations, and organization of a trial. The protocol usually also gives the background and rationale for the trial’3
Clinical protocols are also described as an agreed framework, outlining care to be provided to patients in a designated area of practice.4 They are rigid statements, allowing little or no flexibility or variation, setting out a precise sequence of activities that need to be adhered to when managing an identified clinical condition.5
Clinical protocols are commonly used in clinical trials, such as drug trials, allowing a plan of action to be documented and agreed upon on the basis of best available evidence by a designated group of professionals. Clinical protocols are frequently designed to be concise reference documents, allowing professionals to access quickly agreed methods of action/treatment. In a large organisation clinical protocols can be used to review and improve service quality, providing evidence of actions that the organisation has taken to assure services.6
The NHS Modernisation Agency and National Institute for Health and Clinical Excellence (NICE) promote protocol-based care to give staff greater opportunities to work in new ways and to make the best use of their skills, knowledge and expertise.7 The government bodies say that clinical protocols address the key questions of what should be done, where, when and by whom, maximising the contribution of the multi-disciplinary team (MDT) to patient care. Clinical protocols can assist in removing barriers that only allow doctors or nurses to perform particular types of care.7 Some would argue that the ‘removal of barriers’ justifies the previously quoted anxiety regarding the breaking down of professional practice, as clinical protocols allow unskilled staff to perform tasks that were previously performed by highly skilled and educated staff. However, clinical protocols and guidelines are a reflection of a changing workforce, in which the numbers of highly skilled and educated staff are diminishing, while the workload is rapidly increasing. Therefore, clinical protocols are necessary to support the workers who are increasingly filling these gaps (see Box 21.1). An example of clinical guidance on the development of clinical protocols is the European Medicine Agency guidelines for good clinical practice,3 which define how protocols should be written and followed to ensure international standards are achieved.
Unfortunately, clinical protocols can be perceived as de-personalising care, as they can break down professional practice into chunks of activities. Clinical protocols have been described as negatively affecting individual care management by restricting clinical discretion and by increasing workload as they need regular review. Eventually, they may impact on the need for qualified staff in a variety of care settings.4 Compliance with protocols can be problematic and dissemination needs to be effective.
However, clinical protocols can also have a positive effect as their development can permit staff to feel justified or confident in providing care that was previously seen as an inefficient use of resources6 such as certain drug therapies. Clinical protocols can provide a framework for a complex, specialised sequence of activities and can ensure consensus within a team, contributing towards facilitating change.4
Box 21.1 The benefits of working with protogols to extend a nature’s role8
The NHS Modernisation Agency discussed how the redesign of a nurse’s role into that of a ‘perioperative specialist practitioner’ (PSP) benefits the patient and the surgery and anaesthetic division. The aim is to help reduce waiting lists for inpatient and day surgery by allowing a speeding up of care delivery whilst simultaneously the patients benefit from having dedicated one-to-one care from the PSP. The PSP is able to perform a range of diagnostic and procedural tasks, including many tasks previously carried out by preregistration house officers and senior house officers, by using rigorous protocols that clearly state what the PSP can do, when and where. The PSP is supported legally to practise beyond the original role boundaries.
Guidelines are commonly described as ‘a general rule, principle, or piece of advice’9 However, clinical guidelines are defined as: ‘Systematically developed statements to assist practitioner and patient decisions about appropriate health care for specific clinical circumstances.’10
Clinical guidelines are collated summaries of the current best available evidence regarding clinical efficacy and cost-effectiveness of aspects of healthcare provided to individuals and the general public and the use of technology and interventional procedures in primary and secondary practice. Clinical guidelines are more common than clinical protocols and are intended to help guide an already very busy health professional to provide the most suitable evidence-based care. They don’t replace the health professional’s existing knowledge and skills to make decisions based in individual cases and circumstances. National clinical guidelines aim to ensure that care is consistent and therefore lessen the chance of individual patients being affected by the ‘postcode lottery’ effect if a patient needs a particular, expensive form of treatment.
Clinical guidelines are used by large organisations to develop standards in healthcare and therefore should be of good quality to allow practitioners to be confident when using them.5 They can be used nationally or can be adapted for local use with the inclusion of operational information.10 Clinical guidelines can be used to assist in the assessment of individual health professionals and can also be used as a tool to assist with the ongoing education and training of individuals or groups of healthcare professionals.11
Clinical guidelines are devised by multi-disciplinary groups with the intention of improving the quality of care. There are national and international organisations providing clinical guidance and assistance, such as NICE, Appraisal of Guidelines Research and Evaluation (AGREE), Guidelines International Network (GIN) and the Scottish Intercollegiate Guidelines Network (SIGN) which are used in all areas of healthcare practice, encompassing the professional practice of healthcare professionals and the care they are expected to provide to patients/carers. For example, UK-based nurses are governed by the Nursing and Midwifery Council (NMC) and their unions, such as the Royal College of Nursing (RCN), who provide clinical guidelines on subjects such as wound care, record keeping and fitness to practice. NICE12 have recently released an updated ‘Guidelines Manual’ offering advice to its guideline developers on the processes that should be undertaken in the production of guidance. The AGREE Instrument13 was designed by an international collaboration of researchers and policy makers to improve the quality and effectiveness of clinical practice guidelines in any disease area, including those for diagnosis, health promotion, treatment or interventions. AGREE promote use of the instrument to provide a framework for assessing the quality of clinical practice guidelines developed by local, regional, national or international groups or affiliated governmental organisations.
NICE11 describe their clinical guidelines as ‘recommendations on the appropriate treatment and care of people with specific diseases and conditions within the NHS’. The GIN website14 states that their aim is to promote clinical guidelines through supporting international collaboration of large organisations. It has a database of thousands of guidelines that members can access to review existing international guidelines. SIGN15 aim to improve the quality of healthcare for patients by reducing variation in practice and outcome. They do this through the development and dissemination of national clinical guidelines, containing recommendations for effective practice that are based on current evidence.
Box 21.2 Five Key reasons for choostng an area in which to decelop clinical guidelines16
1. Where there is excessive morbidity, disability or mortality
2. Where treatment offers good potential for reducing moridity, disability or mortality
3. Where there is wide countrywide variation in clinical practice
4. Where resources involved are resource intensive – either high volume and low cost or low volume and high cost
5. Where boundary issues are involved, across sector and across professional boundaries.
Five key reasons for choosing an area in which to develop clinical guidelines16 have been identified in Box 21.2. However, guidelines that have cost-reduction at the heart of their rationale or pay no attention to the level of resources required may not always be an appropriate basis on which a health professional can be expected to make a clinical decision. But they can be used as a tool to help the healthcare professional to make a decision according to the best available evidence.5
Clinical guidelines that impact the POA specialism are the NICE guidelines17 regarding the use of routine preoperative tests for patients having elective surgery. The clinical guidelines offer advice on which tests are relevant, depending on the health status of the individual patient and type of planned surgical procedure. Evidence was collected to examine the implications of ordering or omitting preoperative tests such as blood tests, X-rays and ECGs, from patients who were apparently healthy. The outcome was to offer guidance to reduce the number of tests performed following the preoperative assessment of healthy patients. Clinical guidelines produced by the NHS Modernisation Agency18,19 offer guidance regarding the who, where, what, when and how of setting up preoperative assessment services for inpatients and day surgery.
Clinical protocols and clinical guidelines can be integrated to operate together. Protocols can be a functional part of a clinical guideline or integrated care pathway (ICP), advising on topics such as administration of IV medication, or X-ray procedures.
Legal aspects of guidelines and protocols
The law does not distinguish between definitions of clinical protocols and clinical guidance, or any other statements of clinical guidance, as they share the same general meaning as statements of advice to healthcare professionals on how to proceed in particular circumstances.20
Clinical guidelines can be used in a court of law as evidence of accepted and customary standards of care by the defendant or prosecution during trials examining claims of negligence. They can demonstrate if a healthcare professional has deviated from or adhered to an accepted standard of practice, providing the courts with a benchmark by which to evaluate clinical judgement, but they do not actually set legal standards for clinical care.21 However, clinical guidelines are unlikely to be accepted as a ‘gold standard’ of care as the specific details of individual case circumstances will also need to be taken into account.22 The Bolam test is a standard test of negligence, allowing a court to measure the defendant’s actions against that of an average person. But because healthcare professionals have more than average abilities, the court measures them against the experts in their fields of expertise. Therefore, it is the testimony of an expert witness that the courts draw on to establish accepted and proper practice in specific individual cases, not the standards within a set of guidelines.22
The authors of clinical guidelines do not have a standardised duty of care; ultimately it is the responsibility of the healthcare professional using the guidelines to be aware of this when they choose to utilise the guidance.22 There are known cases where the judge has considered the guideline development process;23 therefore documentation of discussions and meetings is essential to provide evidence of the consultation process between the healthcare professionals involved. The courts will consider whether the clinical guidelines are reasonable, so it is important that they reflect the opinions of a responsible body of healthcare professionals, even if there is another group of healthcare professionals who consider a different method of treatment/care is equally reasonable.23
In 1997, clinical guidelines were predicted to improve the quality of record keeping and communication between staff and patients23 as, in the case of Integrated Care Pathways, the processes of individual care would be signed for when completed and any variance from the norm documented for all the healthcare professionals involved to comment or act upon. Poor communication within the NHS caused 9% of complaints from patients in 2005/6. Of the 95,047 complaints, 8,962 were related to written and oral communication/information given to patients.24 It was the same percentage in 2002/3,25 which could be interpreted as reassuring as there has been no increase but can also be noted as disappointing as there has been no reduction either. It is vital for healthcare professionals to be aware that all documentation can be used in a court of law as evidence in negligence cases; therefore it is important to document care given, protocols and guidance used and any variance from the predicted pathway of care.
Making effective guidelines
Effective guidelines are embedded in practice and their use is sustained by their being a helpful tool for the clinical teams that are using them. It is all too easy to generate guidance which, although well researched, has no chance of implementation in the areas that it is designed for. Guidelines must be relevant enough to be ‘owned’ by those people using them.6
Schwartz et al.1 argue that good guidelines need to be judged against the following criteria:
• Face credibility: This describes the credibility of the authors/organisation behind the guidelines in the eyes of the target group. For a national guideline, it is important that the organisation(s) producing them be respected by the target audience. For example, an audience of anaesthetists is more likely to take note of fasting guidance from the Royal College of Nursing26 because there has been involvement and endorsement by the Royal College of Anaesthetists. (And nurses are more likely to take note of the guidance since it is endorsed by their college.) For a local guideline, the group working on the guideline needs credibility amongst all parts of the target audience.
• Validity: Guidelines can be seen to be valid if their adoption results in better outcomes. This is an argument for audit of guideline adoption, and reminds us that new ways of working are often evaluated well with a Plan, Do, Study, Act (PDSA) cycle, where a new change is planned and implemented, evaluated and then modified or discarded as appropriate. In many circumstances, the validity initially comes from clear evaluation of all (not selected) evidence. Systematic review is a tool used to evaluate and summarise evidence from the literature.27 Criteria are outlined prospectively that are used to identify relevant studies, while rejecting those with poor methodology. This reduces one aspect of bias that is possible in a conventional review, but authors must still be aware of the potential for unregistered negative trials and of the potential for duplication of positive data in several trials. Once the guideline writers have evaluated the quality of the evidence, they should in turn make clear to the audience the basis for their recommendations. Harbour and Miller, on behalf of SIGN,28 advocate a system of grades of recommendation for guidelines depending on the evidence base available. The availability of good-quality evidence can be particularly challenging in the field of preoperative assessment, where there is often a paucity of good-quality evidence on exactly what to do (note that the NICE guidance on preoperative tests, for example,17 is based mainly on level IV evidence, expert opinion).
• Reproducibility: Since valid guidelines should be developed with robust evaluation of all available evidence, the users of guidelines have an expectation that different organisations should come up with similar guidelines. Where two organisations publish different guidance there is potential for a loss of faith in the guideline process. It was heartening to see that the British Hypertension Society and NICE produced common guidance on management of hypertension in 2006,29 after a period of two years when guidance had not been seen to be entirely consistent between the two bodies. One of the best examples of this in the field of preoperative assessment is in the area of planning surgery for patients on warfarin, where there are many local guidelines available, and where close scrutiny reveals slightly differing approaches to the problem.30–32
• Representativeness: People developing guidelines are trusted to produce objective guidance that is free of vested interests. They need to be representative of the groups who are expected to follow such guidance, and be seen as people who are well practised in dealing with the everyday challenges of delivery of care.
• Clinical applicability and flexibility: Rigid guidance that takes no account of local circumstances is less likely to succeed than a more pragmatic approach that takes account of how best to use local resources. Sometimes the best solution nationally may not apply where there are unusual local circumstances.
• Clarity and reliability: It is essential that the messages intended by the authors are conveyed to the guideline users without ambiguity, and that the guideline is clear, readable and easy to follow.
• Transparency: It should be clear how and why the conclusions of the guideline have been reached.
• Scheduled review: The ongoing care of clinical guidelines and clinical protocols is the same; they need regular reviews as promoted by the AGREE instrument, which states that all clinical guidelines should have agreed procedures for updating. This is important as it encourages the developers to investigate whether new evidence regarding treatment and new clinical techniques have become available, reinforcing the strength of the clinical guideline. Such a guideline review offers the opportunity to evaluate implementation of the guideline. Following the decision to use a clinical guideline, the date recommended for update of the clinical guideline should be noted, as it may be due for review and the time and effort for all involved in implementation may be wasted if the updated version is going to change practice again.6 Some guidelines and protocols may be dependent on the number of existing healthcare professionals available. If this, or the availability of equipment and resources changes, then the clinical guidelines or protocols will need updating to ensure that they remain safe.22
The final challenge – getting guidelines into practice and the role of ICPs
If guidelines are developed with these recommendations in mind, there is a chance that they will come to be respected and ‘owned’ by all healthcare professionals making use of them. This is possibly the biggest challenge, to produce guidelines that are used in practice and affect outcomes. The first step has just been described: to make guidelines in such ways that people really want to use them. But even the best guidelines can be left on the shelf if the use of the guidelines is not embedded in practice. This is where ICPs come in.
Lady Thatcher’s opening quote reminds us that use (or otherwise) of guidelines is now endemic through the whole of society to aid decision-making in complex areas. In industry the pioneers of mass production realised early on that a consistent process was important in producing a top-quality product for the cheapest price. ‘Industrial engineering’ started appearing as an academic pursuit in the late 18th century and revolutionised the way that manual workers worked to improve overall efficiency by laying out maps of the optimal processes that then had to be followed.33
More technical tasks proved to be more difficult to prescribe for, considering both the need for complex decision-making and the perceived challenge to the autonomy of a professional workforce. However, even within healthcare Frank Gilbreth was able to influence the more efficient functioning of the surgical team by advocating that the scrub nurse act as ‘caddy’ to the surgeon by passing instruments required on demand!34
The fields of process mapping and the use of standard operating procedures began to influence more complex areas in industry through the 20th century, but healthcare seemed to lag behind until the 1980s. Some clinicians in the USA developed pathways as a means of demonstrating cost effective care and measurable outcomes to insurers, and interest in these concepts spread across the Atlantic over the next decade.35
The preceding paragraphs have described how processes can be developed that embed guidelines and best practice into the routine, whether in industry or in healthcare. But healthcare is a complex area, and there may sometimes be valid reasons to deviate from the prescribed pathway. For example, it is probably quite rational in general that a patient be mobilised on the first postoperative day after a total hip replacement, but it is equally rational that this should not take place if a patient is profoundly hypotensive. Crucially an ICP records such deviations from planned care as variances, and these variances can be audited.
If audit reveals that variances occur frequently, there is an opportunity to study the variances in more detail to determine whether any changes to staff training, resources or the pathway itself are required to improve the service, or whether the presence of a variance might simply be a marker of responsible health professionals making sound judgements rather than simply following a rule book.
In their short history, ICPs have had many definitions and many advocates. Venture Training and Consultancy, a company specialising in ICP support and development, produced this definition in 2002:
An ICP is a document that describes the process for a discrete element of service. It sets out anticipated, evidence based, best practice and outcomes that are locally agreed and that reflect a patient centred, multi-disciplinary, multi-agency approach. The ICP document is structured around the unique ICP Variance Tracking tool. When used with a patient/client, the ICP document becomes all or part of the contemporaneous patient/clinical record, where both completed activities and outcomes, and variations between planned and actual activities and outcomes, are recorded at the point of delivery.35
This definition reminds us that ICPs incorporate and encourage adherence to protocols and guidelines of best practice. They become a means of clinical record keeping that not only makes records more structured and accessible to the multi-disciplinary team, but also has a built-in audit tool to evaluate compliance with the planned process of care.
One of the characteristics of POA is that it does need to be tailored to the needs of the client. Undoubtedly there are common elements to any POA, in terms of the questions asked and the physiological observations made, but clearly guidelines have to help the professionals to extract the correct information, deliver the appropriate advice and document it clearly. Examples in POA practice will be discussed later.
The POA healthcare professional has many roles, and guidance is needed to aid assessment and optimisation of fitness, health promotion, information giving and planning for the perioperative period. Each of these areas encompasses a multitude of guidelines, so the challenge in producing the sheer quantity of guidance cannot be underestimated.
How POA clinical protocols and clinical guidelines were developed in Halifax
Earlier in this chapter we discussed the possible reasons why guidelines might be developed. In this case guidelines were required to support a significant reform of the preoperative assessment process, moving from a somewhat inconsistent process run with junior doctors and ward nurses to one where dedicated preoperative assessment nurses would work with sessional consultant anaesthetic support to provide a more consistent process. It was anticipated that such a process would reduce on day cancellations, improve patient optimisation for surgery, reduce the time taken for anaesthetic and surgical assessments on arrival and therefore facilitate same day admission. Happily it has achieved these aims.
Since there was a wholesale change in process, we had a unique opportunity to develop paperwork that fitted into our new process, and to design documentation that automatically referred to other guidance, such that any guidelines that we make are automatically embedded in practice.
Having a number of integrated care pathways already in existence in our organisation, it was logical to use this as a tool, using the preoperative section of a total hip replacement pathway as a starting point. This had the added advantage that the broad appearance and function of the document would be familiar to nursing and medical staff.
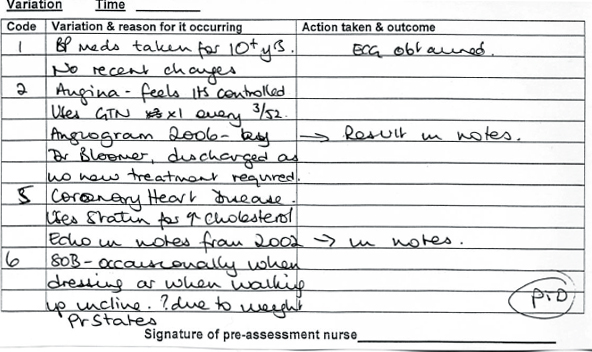
The Calderdale and Huddersfield POA ICP (see Appendix, pages 395–406) works with some accompanying notes that describe actions to be taken in response to information obtained from patient or notes. The POA ICP was designed to accommodate the multi-disciplinary team (MDT) who would use the pathway in the POA unit and on admission. It was divided into sections for ease of reference; a quick referral sheet was developed for use by the nursing staff that combined demographic details with a short medical/surgical history and social history. A section is then allocated for the POA nurse to identify MDT members the patient has been referred to such as occupational therapist, physiotherapist, social services or the dietician. The ICP also includes a section containing a list of health conditions with a tick box history sheet, which, at a glance, identifies the health conditions of the patient (see Figure 21.1, page 390). If a problem is identified, a tick would appear in the ‘yes’ box and this is flagged as a variance on a variance list (see Figure 21.2, page 390).
The problem is expanded on in line with a guideline and an action to be taken is documented in the light of this information.
So in the Halifax POA ICP, a completely healthy person will have no variances listed in the variance box that follows the medical history tick list.
This means that for the medical assessment parts of the pathway (but not the investigations done or information given parts) a variance demonstrates a deviation of the patient from the norm, rather than a deviation from the planned pathway of care. This approach is probably not for the ICP purists, but is a pragmatic approach based on the fact that POA patients have a variety of co-morbidities and no two patients are the same. Since these variances can easily be compared with the guidelines that are structured around the ICP, we believe that we still have the ability to compare the care that should be planned, with that actually delivered. This system of care facilitates variance tracking and audit.
By performing POA early in the surgical journey, we are able to ensure that only fit patients are on surgical waiting lists and comply with the UK Government commitment on surgical waiting times. The timing of the patient’s POA appointment had an impact on the design of the POA ICP. We arranged for the appointment to take place soon after the decision to operate was made; therefore a further assessment is necessary before the patient is admitted for surgery. A telephone assessment was incorporated within the ICP, and this takes place up to four weeks prior to admission to ensure there have been no changes to the patient’s health since the initial assessment. The POA appointment is also an appropriate time to arrange preoperative tests or special admission arrangements, so a check list was developed to allow the POA nurse to identify, at the original POA appointment, any preoperative preparation required, which could then be referred to at the later telephone assessment.
The accompanying notes and POA ICP incorporate health promotion as information regarding smoking, alcohol intake and illicit drug use is not only requested from all patients but it is also acted upon. The guidelines advise accordingly, regarding excessive use of alcohol, smoking cessation and weight loss. Further assistance is offered in the form of referrals to health specialists such as dieticians and smoking cessation and documented in the POA ICP so each MDT member in contact with the patient is aware of previous discussions and can follow up to review the referrals.
The POA ICP has an MDT section for the anaesthetists to document actions about patients reviewed in the anaesthesia clinic. There are two anaesthetic clinics a week, attended by patients referred to the clinic by the POA nurses due to surgery type (for example, major vascular or abdominal surgery) or uncontrolled or undiagnosed health conditions. Following these appointments, the consultant commonly dictates a letter which is later filed within this section. The anaesthetists also review the ICPs of patients where the POA nurses have concerns, such as patients with complicated but controlled medical histories or unusual diseases. The anaesthetists review all abnormal ECGs and blood tests; therefore a tick box was incorporated to allow the doctors to quickly note if the test was abnormal and needed action. Again, if a letter is generated to a GP, then a copy of it is placed in this section for the MDT, promoting communication between all concerned parties. The MDT section is also used by the POA nurses in the event that a patient is deemed unfit for surgery. The continuing activities that assist the nurses to ensure the patient is eventually safely admitted for planned surgery are documented here.
The POA ICP continues to evolve. For example, originally a smaller variance list was used which did not accommodate the amount of information each health condition required, even if the patient didn’t have a complicated health history. This was made twice the size by the ICP facilitator. Also, the brief information regarding surgical and medical history for the ward nurses was originally on separate pages but these were time-consuming to complete and refer to, so the information is now combined in one box on the same page.
This continued evolution of the ICP has been informed by surveys of the POA ICP to assess how various members of the MDT feel about the documentation. Audits have been performed to assess completion of the ICP by the POA MDT and feedback given to the team in order to improve performance and further modify the document. The POA ICP has been used to audit preoperative testing, providing evidence that assists the team in identifying how to reduce costs and identify sections of the accompanying notes in need of updating. Evaluation of the effect of adherence to the guidance has been performed from the perspective of patients and from the perspective of ward nurses and allied health professionals, surgeons, and anaesthetists, and has produced evidence of the resulting improvements in patient preparation and reduced operative cancellations.
Figure 21.3 Example of medical condition list
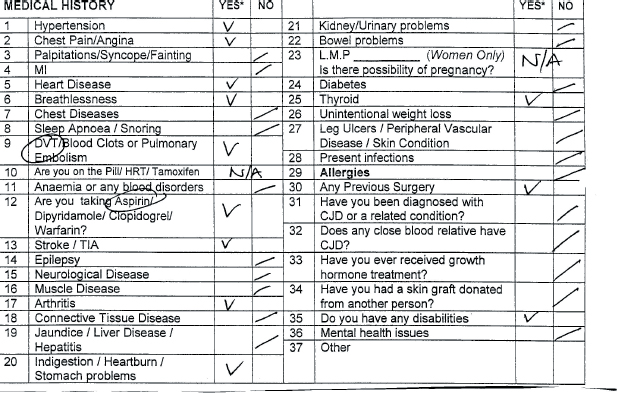
Figure 21.4
Examples of variance list expanding on health conditions and leading to actions

CONCLUSION
While guidelines are now a common part of everyday life, they are absolutely crucial in a complex area such as POA. Guidelines should be made using the best evidence available, be applicable to local circumstance and be subject to regular review. However, unless the use of guidance is embedded in practice, compliance may be low. In the wider world, this problem is often solved by development of rigid processes, designed to increase productivity and efficacy and in some industries improve quality. In healthcare, the use of integrated care pathways can help improve the quality of care by promoting compliance with guidelines and by facilitating audit of the process, while still leaving scope for clinical judgement.
REFERENCES
1. R. Scott (1996). Report of the enquiry into the Export of Defence Equipment and Dual-use goods to Iraq and Related Prosecutions. London: HMSO. In P.J. Schwartz, G. Breithardt, A.J. Howard, D.G. Julian and N. Rehnqvist Ahlberg (1999). The legal implications of medical guidelines – a Task Force of the European Society of Cardiology. European Heart Journal 20: 1152–7.
2. American Heritage Dictionary (2007). http://www.Answers.com/topic/protocol (accessed 22.3.2007).
3. European Medicines Agency (2002). Guideline for Good Clinical Practice.
http://www.emea.eu.int/pdfs/human/ich/013595en.pdf (accessed 10.04.07).
4. NHS Working in Partnership Programme (2006). Using Clinical Protocols, Standards, Policies and Guidelines to Enhance Confidence and Career Development.
http://www.wipp.nhs.uk/uploads/GPN%20tools/Tool5.8-UsingProtocols.pdf (accessed 12.04.2007).
5. R. Broughton and B. Rathbone (2001). What makes a good clinical guideline? Evidence Based Medicine 1(11).
www.evidence-based-medicine.co.uk (accessed 12.04.2007).
6. J. Hewitt-Taylor (2006). Clinical Guidelines and Care Protocols. Chichester: John Wiley.
7. NHS Modernisation Agency and NICE (2004). What is Protocol-based Care?
http://www.modern.nhs.uk/protocolbasedcare/whatis_leaflet.pdf (accessed 14.04.07).
8. NHS Modernisation Agency (2005). Protocol Redesign – Some Case Studies and Examples. London: Department of Health.
http://www.wise.nhs.uk/PBCIP/br_role-redesign.pdf (accessed 14.04.07).
9. AskOxford.com (2007). On line dictionary. http://www.askoxford.com (accessed 17.04.07).
10. M.J. Field and K.N. Lohr (1992). Guidelines for Clinical Practice: From Development to Use. Institute of Medicine, Washington, DC: National Academy Press.
11. National Institute for Health and Clinical Excellence (NICE) (2005). A Guide to NICE. London: NICE.
http://www.nice.org.uk/page.aspx?o=guidetonice (accessed 17.04.07).
12. National Institute for Health and Clinical Excellence (NICE) (2007). A Guidelines Manual. London: NICE.
http://www.nice.org.uk/page.aspx?o=guidelinesmanual (accessed 25.04.07).
13. Appraisal of Guidelines Research and Evaluation (AGREE) Research Trust (2004). The AGREE Instrument.
http://www.agreetrust.org/docs/AGREE_Instrument_English.pdf (accessed 28.04.07).
14. Guidelines International Network (G-I-N) (2007). http://www.g-i-n.net/index.cfm?fuseaction=about (accessed 28.04.07).
15. The Scottish Intercollegiate Guidelines Network (SIGN) (2007).
http://www.sign.ac.uk/about/introduction.html (accessed 28.04.07).
16. NHS Executive (1996). Clinical guidelines: using clinical guidelines to improve patient care within the NHS. Leeds: NHS Executive. Cited in Health Management Specialist Library (2007). Clinical guidelines: A brief introduction. NHS National Library for Health. http://www.library.nhs.uk/healthmanagement (accessed 22.03.07).
17. National Institute of Clinical Excellence (NICE) (2003). Preoperative Tests: The Use of Routine Preoperative Tests for Elective Surgery. London: NICE. http://guidance.nice.org.uk/CG3 (accessed 26.04.07).
18. NHS Modernisation Agency Operating Theatre and Pre-operative Assessment Programme (2003). National Good Practice Guidance on Preoperative Assessment for Inpatient Surgery. London: Department of Health.
19. NHS Modernisation Agency Operating Theatre and Pre-operative Assessment Programme (2001). National Good Practice Guidance on Preoperative Assessment for Day Surgery. London: Department of Health.
20. B. Hurwitz (1998). Clinical Guidelines and the Law – Negligence, Discretion and Judgment. Oxford: Radcliffe Medical Press.
21. B. Hurwitz (2004). How does evidence based guidance influence determinations of medical negligence? British Journal of Medicine 329: 1024–8.
22. B. Hurwitz (2000). Clinical practice guidelines: legal, political and emotional considerations. In M. Eccles and J. Grimshaw (eds) Clinical Guidelines from Conception to Use. Oxford: Radcliffe Medical Press.
23. J. Tingle 1997). Clinical guidelines and the law. In J. Wilson (ed.) Integrated Care Management the Path to Success? Oxford: Butterworth-Heinemann.
24. The Information Centre (2006). Data on Written Complaints in the NHS 2005-06
http://www.ic.nhs.uk/webfiles/publications/nhscomplaints/WrittenComplaintsNHS151106_XLS.xls (accessed 6.05.07).
25. Department of Health (2003). Written complaints about hospital and community services by service area, England, 2002-03
http://www.performance.doh.gov.uk/hospitalactivity/data_requests/download/nhs_complaints/complaint_03_summary.xls (accessed 14.05.07).
26. Royal College of Nursing (2005). Perioperative fasting in adults and children 2005.
http://www.rcn.org.uk/publications/pdf/guidelines/PerioperativeFastingAdultsandChildren (accessed 12.06.07).
27. T. Greenhalgh (1997). How to read a paper: Papers that summarise other papers (systematic reviews and meta-analyses) British Medical Journal 315: 672–5.
28. R. Harbour and J. Miller (2001). A new system for grading recommendations in evidence based guidelines. British Medical Journal 323: 334–6.
29. National Institute for Health and Clinical Excellence (2006). Hypertension: management of hypertension in adults in primary care 2006 http://guidance.nice.org.uk/CG34 (accessed 12.06.07).
30. South Devon Healthcare NHS Trust (2005). Perioperative guideline for patients on Warfarin 2005.
31. Heart of England NHS Trust (2006). Peri-op Warfarin guideline.
32. Calderdale and Huddersfield NHS Trust (2007). Guideline for the perioperative management of anticoagulation in Warfarinised Patients 2007.
33. Wikipedia. ‘Industrial engineering.’ http://en.wikipedia.org/wiki/Industrial_engineering (accessed 12.05.07).
34. Wikipedia. ‘Frank Gilbreth.’ http://en.wikipedia.org/wiki/Frank_Gilbreth (accessed 12.05.07).
35. National Library for Health. Protocols and care pathways.
http://healthguides.mapofmedicine.com/choices/map/index.html (accessed 9.10.10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





