57 Congestive Heart Failure
• Acute decompensated heart failure can be manifested as volume overload, acute diastolic dysfunction, and low cardiac output.
• Identifying and addressing the precipitants of the decompensation are as important as treating the decompensation itself.
• Biomarkers such as B-type natriuretic peptide and the inactive N-terminal fragment of B-type natriuretic peptide can assist in making the diagnosis, but it is important to understand the limitations of these tests.
• In patients with volume overload, diuretics remain the cornerstone of therapy.
• Not all patients with acute decompensated heart failure are significantly volume-overloaded; overaggressive diuresis risks hypotension and worsening renal function.
• Nitrates are first-line therapy for patients in whom an acute reduction in cardiac preload and afterload is desired.
• Inotropic therapy should not be routinely instituted unless the patient is in cardiogenic shock.
• For patients in respiratory distress, noninvasive support (continuous positive airway pressure or bilevel positive airway pressure) may reduce the need for endotracheal intubation.
Epidemiology
With the aging of the U.S. population and improved survival after myocardial infarction, the prevalence of heart failure is on the rise.1 At the same time, advances in medical therapy are allowing patients with heart failure to live longer. In 2008, 5.7 million Americans were estimated to have heart failure, with approximately 670,000 new cases diagnosed that year. Heart failure contributes to nearly 300,000 deaths per year, and costs associated with the treatment of heart failure exceed $30 billion annually. Heart failure accounts for nearly 1 million inpatient admissions per year and represents the primary reason for hospitalization in the growing elderly population. Approximately four of every five patients hospitalized for heart failure initially come to the emergency department (ED) for treatment.
Evidence-based literature for ED management of heart failure lags behind that of other emergency conditions, such as acute coronary syndrome and stroke. The number of large, randomized controlled clinical trials is small, and most practice guidelines, such as those from the Heart Failure Society of America and the European Society of Cardiology, rely heavily on consensus statements.2,3 A recent American Heart Association scientific statement highlighted the significant gaps in knowledge and the lack of evidence-based approaches to the management of heart failure in the ED.4 In contrast, data from the Acute Decompensated Heart Failure National Registry (ADHERE) and the Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) registry have provided important insight into the clinical characteristics and actual patterns of care of these patients.5,6
In addition to the paucity of controlled clinical trial data, there remains confusion about terminology. Heart failure refers to the clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood. Causes of chronic heart failure are numerous and diverse (Box 57.1), but in the United States the majority of cases arise as a consequence of coronary artery disease and long-standing hypertension. The term acute heart failure is reserved for the presence of acute signs and symptoms of heart failure in an individual without previously known structural or functional cardiac disease. Examples of acute heart failure are massive ST-segment elevation myocardial infarction, acute papillary muscle rupture, and fulminant myocarditis. Much more commonly, a patient comes to the ED with worsening symptoms of known chronic heart failure, in common parlance a “heart failure exacerbation.” The term acute decompensated heart failure (ADHF) has been adopted to describe this phenomenon, whereby a patient with an established diagnosis of heart failure experiences increasing signs and symptoms of the disease after a period of relative stability.
Pathophysiology
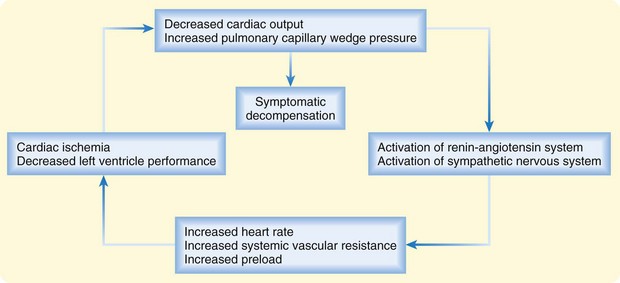
In patients with chronic heart failure, inadequacy of cardiac function sets in motion a common set of compensatory mechanisms based on the Frank-Starling relationship and characterized by elevated sympathetic tone, fluid and salt retention, and ventricular remodeling. These adaptations can allow heart failure to remain stable (or “compensated”) for a time but also provide the final common pathway for decompensation—a downward spiral that can accelerate in response to a particular precipitant or stress (Fig. 57.1). High circulating levels of aldosterone, vasopressin, epinephrine, and norepinephrine can become maladaptive when tachycardia and vasoconstriction compromise intrinsic left ventricular (LV) performance and worsen myocardial oxygen balance. Deteriorating LV function can result in further neurohormonal activation and self-perpetuation of this adverse cycle. Acute decompensation can develop over a period of minutes, hours, or days and can range in severity from mild symptoms of volume overload or decreased cardiac output to frank pulmonary edema or cardiogenic shock.
Although ADHF represents a final common pathway, it is generally triggered by one or more specific precipitants (Box 57.2). Noncompliance with medications or dietary restrictions and myocardial ischemia are believed to be the most common causes of clinical cardiac decompensation. Other cardiovascular precipitants are arrhythmia (atrial fibrillation in particular), acute valvular dysfunction, and hypertensive crisis, but ADHF can also arise as a consequence of noncardiac conditions such as infections, anemia, alcohol withdrawal, uncontrolled diabetes, and thyroid disease.
Presenting Signs and Symptoms
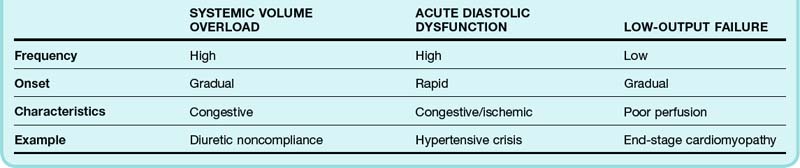
The heterogeneity of the signs and symptoms in patients with ADHF reflects, to some extent, the relative contributions of volume overload, acute diastolic dysfunction, and low cardiac output (Table 57.1). Volume overload, which usually occurs in the setting of medication noncompliance or dietary indiscretion (or both), is classically associated with gradually worsening congestive symptoms. Acute diastolic dysfunction can occur in the setting of myocardial ischemia, tachyarrhythmia, or uncontrolled hypertension and is typically manifested as flash pulmonary edema. Nearly half of all patients admitted to the hospital for ADHF have mild or no impairment in systolic function.5 Overt manifestations of low cardiac output (i.e., hypoperfusion) are not generally seen except in patients with advanced LV dysfunction.
Most patients with ADHF have some degree of dyspnea. However, ADHF can closely mimic many other cardiac, respiratory, and systemic diseases. Historical features such as a history of orthopnea, paroxysmal nocturnal dyspnea, or peripheral edema make the diagnosis of ADHF more likely. The most valuable single piece of historical information to elicit from patients is a previous history of heart failure, myocardial infarction, or coronary artery disease. For example, patients evaluated in the ED because of acute dyspnea are approximately six times more likely to have ADHF if they have previously experienced heart failure (Table 57.2).7
Table 57.2 Sensitivity, Specificity, and Positive Likelihood Ratio of Selected Clinical Findings Associated with Acute Decompensated Heart Failure
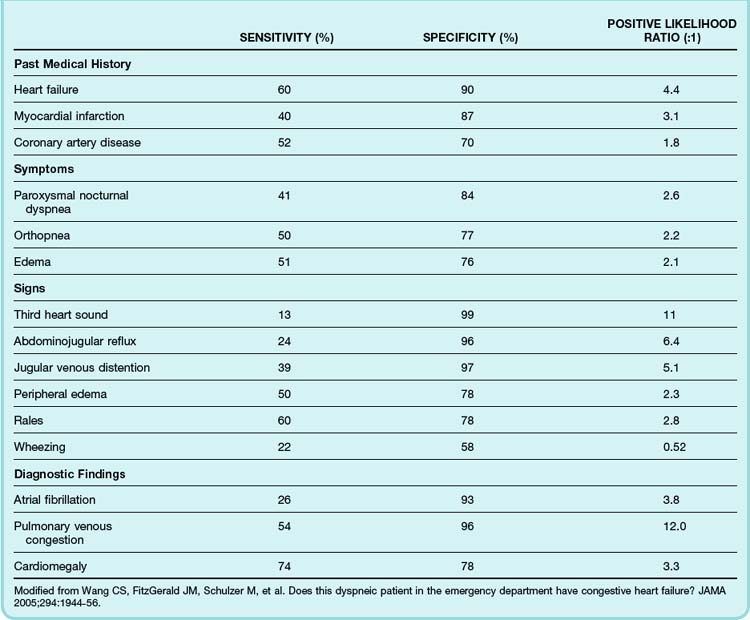
The diagnostic utility of the physical examination has been well studied in the setting of chronic heart failure but less so for ADHF. It should be recognized that in ADHF, physical findings may be misleading because of the rapidly evolving clinical situation. Generally speaking, jugular venous distention, abdominojugular reflux, pedal edema, and an audible third heart sound are specific but insensitive indicators of heart failure, whereas the presence of pulmonary rales has only moderate specificity for heart failure (see Table 57.2).7
Differential Diagnosis and Medical Decision Making
Even before patients reach the hospital, ADHF is associated with significant morbidity and mortality, including malignant arrhythmias and prehospital cardiac arrest. With few exceptions, the safety and efficacy of prehospital interventions have been poorly studied. Prehospital therapy for decompensated heart failure should be undertaken with caution in light of the relatively high number of inaccurate diagnoses made in the field. In as many as 50% of patients with assumed heart-associated respiratory distress, a different condition is diagnosed once they arrive at the hospital. Despite these concerns, evidence suggests that prehospital therapy for presumed heart failure can prevent serious complications and improve survival, particularly for critically ill patients. For example, prehospital use of continuous positive airway pressure (CPAP) in patients with acute pulmonary edema may avert the need for endotracheal intubation.8
Nitroglycerin appears to be the safest and most effective of the prehospital medications used for presumed pulmonary edema.9 The role of other medications for heart failure in the prehospital setting is less clear. Early administration of furosemide appears to have very little benefit and may result in short-term complications. Prehospital use of morphine sulfate for presumed pulmonary edema has been associated with a higher rate of endotracheal intubation, particularly in patients whose condition turns out to have been misdiagnosed in the field.
Diagnostic Studies
B-Type Natriuretic Peptide and NT-Probnp
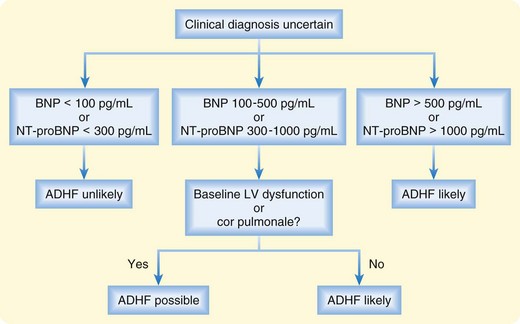
Plasma levels of BNP and NT-proBNP have been shown to be useful in distinguishing between cardiac and noncardiac causes of dyspnea.10,11 Acutely dyspneic patients with plasma BNP levels lower than 100 pg/mL or NT-proBNP levels lower than 300 pg/mL are very unlikely to have ADHF (90% to 99% sensitivity), whereas those with BNP levels higher than 500 pg/mL or NT-proBNP levels higher than 1000 pg/mL are very likely to have ADHF (87% to 95% specificity). Intermediate levels must be interpreted in the clinical context (Fig. 57.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree