Fig. 12.1
A transverse section through a typical thoracic dermatome at the level of the intervertebral foramen (Modified from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)
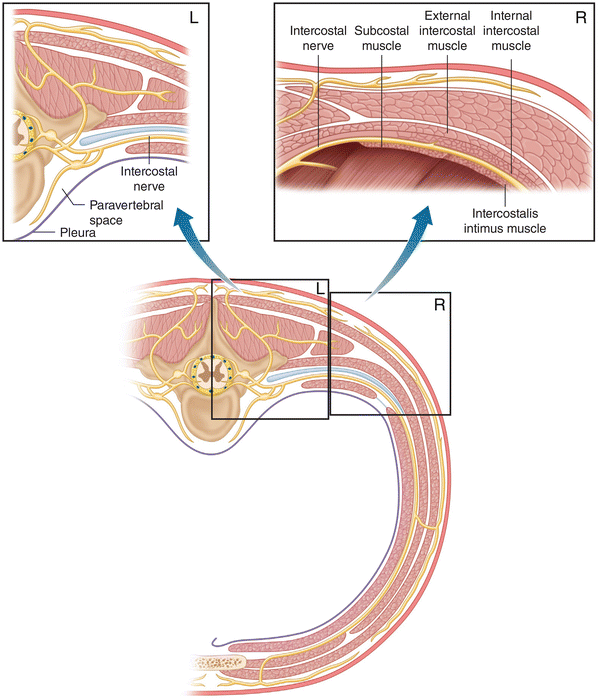
Fig. 12.2
Paravertebral nerve blocks and interpleural nerve blocks act in the area of the left upper and lower boxes. Intercostal nerve blocks are applied to the anatomy depicted in the right upper and lower boxes (Modified from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)
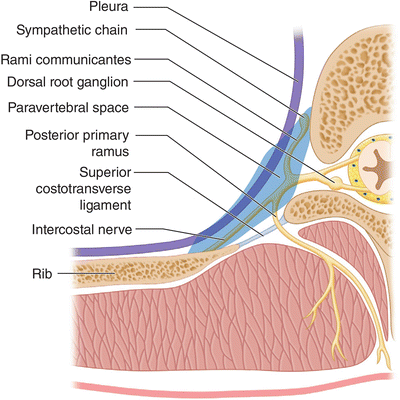
Fig. 12.3
The paravertebral space is defined by four borders: (1) medial, vertebral body; (2) lateral, intercostal space, and the costotransverse ligament; (3) superior, bone, and articular capsules of the rib and transverse process above; and (4) inferior, the rib below. In three dimensions, the space is a four-sided pyramid with its base at the pleura and apex at the intervertebral foramen (Modified from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)
Paravertebral Anatomy
The paravertebral space (Fig. 12.3) is the shape of a four-sided pyramid with its apex facing posteriorly into the neural foramen and its base bordered anteriorly by the parietal pleura. The thoracic paravertebral space is defined by the following four borders: (1) the bone and articular capsules of the rib and transverse process above, (2) the rib below, (3) medially by the vertebral body, and (4) laterally by the intercostal space and the costotransverse ligament. The costotransverse ligament runs from the transverse process to the superior root and its continuation, the intercostal nerve. The intercostal nerve branches into dorsal and ventral rami in the paravertebral space. Gray and white rami communicates course through the space to and from the respective sympathetic ganglion at that level, which is also contained within the paravertebral space. Other contents include areolar tissue, fat, and blood vessels. It is important to keep in mind that the paravertebral space is contiguous with the epidural and intercostal spaces as it lies between these two other spaces. Any substance injected into the paravertebral space may potentially spread cephalad and caudad to adjacent paravertebral spaces as well as medially and laterally to the epidural and intercostal spaces, respectively [56]. Rarely, an injection into the paravertebral space will spread to the contralateral space, and this has been demonstrated radiologically [19–21, 57–59].
In general, topographic spread is variable and difficult to predict [60, 61]. Naja et al. performed a series of paravertebral blocks using nerve stimulator guidance to determine the effect of varying injection points on spread of solution [61]. Their findings indicated that injection in the more ventral aspect of the thoracic paravertebral space resulted in a multisegmental longitudinal spreading pattern. Injecting at the dorsal aspect of the space showed a cloud-like spread with limited distribution to adjacent segments (Fig. 12.4).
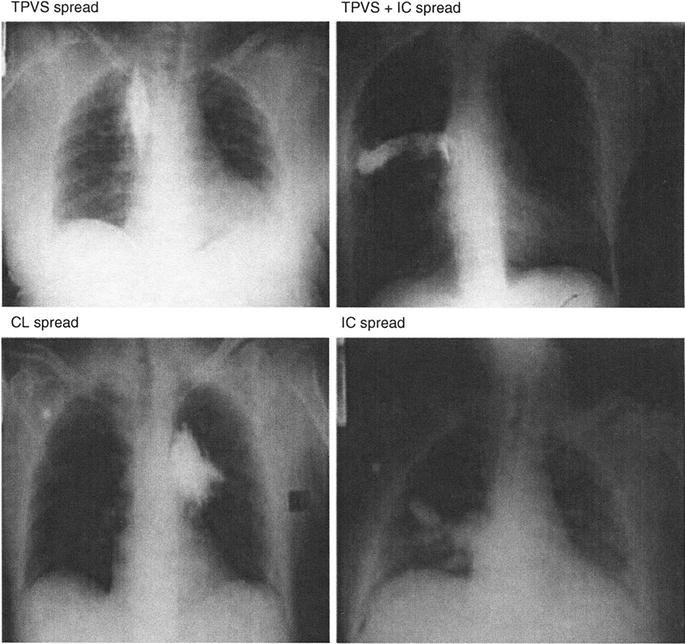

Fig. 12.4
Thoracic paravertebral space (TPVS). TPVS + IC TPVS +Intercostal. CL, cloud like; IC, intercostal (From Naja et al. [46] reprinted with permission from Blackwell Publishing)
The similarity in the anatomic distribution and density of block produced by continuous paravertebral block and continuous epidural infusion would seem to indicate that some cases of unilateral “epidural” block might be attributable to inadvertent continuous paravertebral blockade. This phenomenon has been confirmed radiologically [62].
Paravertebral Nerve Block Technique
Patient comfort during performance of a paravertebral block is improved by good technique, the use of small-gauge needles, and the avoidance of paresthesias while performing the block [63]. Generous infiltration of local anesthetic also makes the procedure more tolerable. Sedation before the procedure is strongly recommended and adds greatly to patient comfort.
Classic Technique—Lateral Approach
The classic technique for paravertebral blockade involves insertion of a needle 4.0 cm lateral to the midline, level to the caudad aspect of the spinous process one level above the level to be blocked (Fig. 12.5). The caudad angle of the thoracic spinous process brings the inferior tip of the spinous process to the superior aspect of the spinous process at the level below [64]. The needle is advanced perpendicular to the skin in all planes until it contacts the transverse process. The depth of the needle is noted. A sterile hemostat can be clamped to the needle to mark the depth of the needle at the skin. The needle is then “walked off” the transverse process in a cephalad direction and advanced 1 cm, placing the tip of the needle in the paravertebral space. Modification of this technique by advancing the needle medially to contact the vertebral body affords relative confidence that an intraneural or subarachnoid injection will not occur. (See detailed description later.) Because the epidural space is contiguous with the paravertebral space via the intervertebral neural foramen, epidural spread is always possible if enough volume is injected.
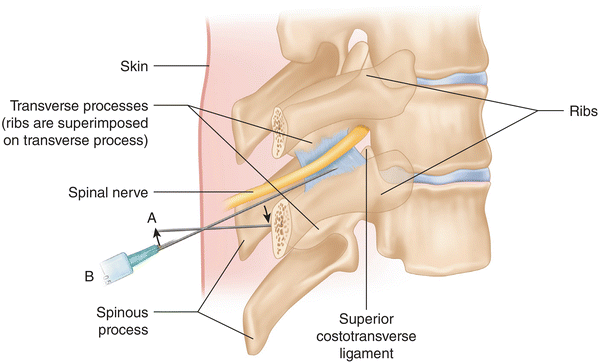

Fig. 12.5
Insert needle 4 cm lateral to the midline at the level of the caudad tip of the spinous process, one segment above the level to be blocked. Advance needle to the transverse process (TP) A. “walk off” TP in a cephalad direction B. Advance needle 1 cm into the PVS (Reprinted from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)
Medial Approach
To avoid intrathecal injection, Shaw recommends a medial approach [65]. The needle insertion point is approximately 1 cm from midline. The needle is advanced until the lamina is contacted and then directed laterally off the bone. With this technique, the tip of the needle is directed away from the neuraxis, but intraneural injection and epidural extravasation are still possible. Tenicela and Pollan modified and strongly advocate performance of the medial approach in the following manner: after a skin wheal is placed, generous infiltration of local anesthetic into the paraspinal muscles is performed 3–4 cm lateral to the midline in the thoracic region and 2–3 cm lateral to midline in the lumbar region [17]. A 22-gauge, 9-cm spinal needle is inserted and advanced at a 45° angle to the transverse plane in a medial direction until the lamina is contacted. The approximate depth required to make contact with the lamina is 5–6 cm in males and somewhat less in females. Gentle aspiration is performed to confirm negative return of blood or cerebrospinal fluid (CSF). At this point, a small amount of local anesthetic is injected at the periosteum. A sterile hemostat is clamped to the shaft of the needle about 1–1.5 cm from the skin, marking the depth of the lamina. The needle is then withdrawn and guided laterally off the lamina and advanced until the hemostat is flush with the skin. After negative aspiration for blood, CSF, and air, a test dose of 3 mL is given. The remaining dose can be given if there was no adverse response to the test dose. If bone is contacted at increasingly superficial levels, the needle has contacted the transverse process and is too cephalad. It must be reinserted approximately 1 cm caudad. These authors claim good to excellent results in 97 % of 380 performances of paravertebral block.
Continuous Technique
Further modification of the injection technique allows placement of a catheter for continuous infusion. Eason and Wyatt proposed that this technique achieves the closest possible approximation of the needle tip with the common intercostal nerve (i.e., before division into dorsal and ventral rami) [66]. By using an epidural needle, a catheter can be advanced for repeated bolus dosing or continuous infusion. Beginning 3 cm lateral to midline, a needle is passed perpendicular to the skin in all planes. The needle is advanced until it contacts bone, which may be rib or transverse process. From this point, the needle is walked cephalad off the bone. This technique was proposed to be safer than using the caudad direction for performance of the block (Fig. 12.6). Loss of resistance with an air-filled syringe is used to identify entrance of the needle tip into the paravertebral space. When the needle is in the costotransverse ligament, there is significant resistance to attempted injection of air. Once the needle tip passes into the loose areolar tissue of the paravertebral space, the air can be injected. If a catheter is advanced, it should have a single orifice at the tip to ensure that aspiration will give accurate information about the location of the tip. An insertion depth of 1 cm is suggested.
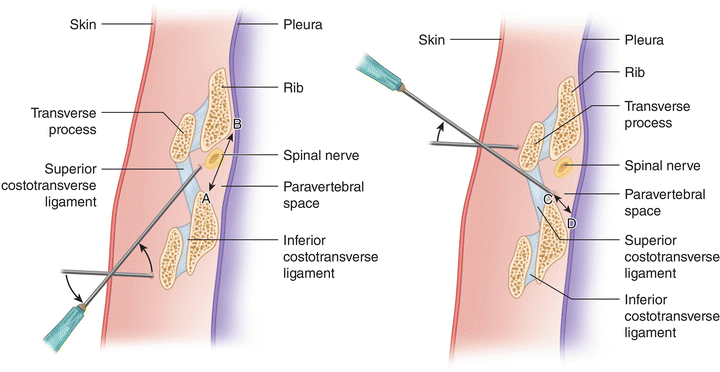

Fig. 12.6
The distance from the superior costotransverse ligament to the pleura is longer with the cranial approach (line a, b) than it is with a caudad approach (line c, d). The risk of pneumothorax may therefore be decreased with a superior approach (Modified from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)
The authors report that manipulation of the epidural needle may be necessary to actually insert the catheter into the paravertebral space. An easily advancing catheter may indicate interpleural localization [57]. Injection of 15 mL of 0.375 % bupivacaine reliably blocks four dermatomes.
Ultrasound-Guided Paravertebral Nerve Block
A linear high-frequency ultrasound transducer is used in the transverse plane with its medial border just lateral to the previously identified spinous process of the vertebral body at the level of the facet joint, and an ultrasound survey scan is obtained. Once the transverse process is identified, the transducer is slowly moved superiorly or inferiorly until the space between the two adjacent transverse processes is identified. One can identify the pleura that appears as a bright hyperechoic downward curving line, which can be seen to slide back and forth with respiration. Just above the hyperechoic pleural line as it curves down as it moves medial toward the vertebral body is the triangular-shaped thoracic paravertebral space. Just above the paravertebral space is the linear hyperechoic internal intercostal membrane. The depth of the posterior border of the paravertebral space is noted. When these anatomic structures are clearly identified on the transverse ultrasound scan, the skin is prepped with anesthetic solution, and a 3 ½ in. needle with stylet is advanced from the middle of the inferior border of the ultrasound transducer using an out-of-plane approach with the trajectory being adjusted under real-time ultrasound guidance until the needle tip is at the previously identified depth of the posterior border of the paravertebral space. After careful aspiration, a small amount of solution can be injected to aid in identification of the needle tip position. The needle is then advanced slowly with attention paid to the relative location of the bright hyperechoic pleura line until the needle tip is seen to be within the paravertebral space. After careful aspiration, the remainder of the solution is slowly injected. The needle is then removed and sterile pressure dressing placed at the injection site [67].
Complications/Treatment
The most important factors for safe performance of paravertebral neural blockade are a solid knowledge of pertinent anatomy, meticulous attention to injection technique, and anticipation of all possible physiologic changes associated with the block. The clinician must have a comprehensive understanding of the potential complications. Early recognition facilitates rapid treatment, thus minimizing more serious sequelae. Utilization of a nerve stimulator-guided technique is associated with a higher success rate and fewer complications than standard techniques [68]. It is strongly suggested that an intravenous line be in place before performing the block.
It is imperative that low-osmolarity contrast agents be used when performing these blocks, because spread of high-osmolarity solutions into the subarachnoid space can lead to significant neurologic harm. The proximity of the paravertebral space to the central nervous system creates the obvious potential for needle entrance into either the epidural or subarachnoid space. Iodinated contrast has been injected into the epidural space with and without spread into the paravertebral space. In performing 45 paravertebral blocks, Purcell-Jones et al. showed contrast confined to the paravertebral space in only 18 % of procedures. There was epidural extravasation in 70 % and exclusive epidural spread in 31 % of cases [56].
In addition to epidural [13] and subdural [69] injection, unrecognized subarachnoid puncture can occur. Headaches not associated with obvious dural puncture occurred in 3 of 24 cases in one series of paravertebral blocks. Aspiration was negative for CSF before injection [70]. Negative aspiration for CSF is not an absolute guarantee of proper needle placement, especially with small-gauge needles or long, small-bore catheters. The headaches resolved with conservative management within 5–14 days postoperatively. The medial approach proposed by Shaw and modified by Tenicela and Pollan has shown excellent results with low complication rates [17, 65]. Of the 384 blocks performed by Tenicela and Pollan, there was one incident of pneumothorax (0.26 %), one recognized dural puncture, two intrathecal injections of the test dose, 18 incidents of hypotension (4.6 %), five bilateral blockades (1.3 %), and 27 incidents of fair to poor block (7.0 %). Poor results were attributed to centralized pain disorders. There were no incidents of serious or permanent sequelae.
Intravenous, intra-arterial, and intraneural injection can occur using any approach to the paravertebral space [68]. In addition, infection, hematoma formation, or damage to the neural fascicle may occur from dry needling. The type of needle can also affect the incidence of sequelae. Short-beveled needles have been shown to cause less nerve damage than long-beveled needles [71].
Aspiration will not reveal the presence of an intrafascicular needle tip. Injectate can dissect back through an epineural injection to the contiguous pia mater [72, 73]. This is driven by the occurrence of severe sequelae (death, paraplegia, transverse myelitis) from injection of a long-acting formulation of procaine [74–76]. The diffuse tissue necrosis was attributed to the carrier solution [77]. For this reason, the use of fluoroscopy and injection of low-osmolarity iodinated contrast to confirm proper needle placement are recommended when performing paravertebral blockade.
When using a continuous technique, there is always a risk of shearing the catheter if it is withdrawn back through the needle. Predictably, there will almost always be some pain at the site of needle insertion. Infection and hematoma are also possible risks. Monoplatythela (unilateral flat nipple) may occur with a successful block [78].
Other potential complications involve interpleural or intrapulmonary injections. If the tip of the needle is in the interpleural space, aspiration should reveal air. Injection of a small volume of radiocontrast under live fluoroscopy can quickly and easily detect an interpleural or intrapulmonary injection.
Prolonged anesthesia and motor block after inguinal hernia repair under general anesthesia with paravertebral blockade was observed in a patient with multiple sclerosis [79]. Abnormal uptake of local anesthetics into the spinal cord secondary to the presence of demyelination was proposed as the mechanism.
Contraindications to paravertebral block are infection at the site, patient refusal, and allergy to any of the solutions to be injected.
Intercostal Nerve Blocks
Intercostal nerve blocks are an alternative to thoracic epidural analgesia. Intercostal nerve blocks can be used in the acute setting for rib fractures, postthoracotomy pain, and trauma. Potential pitfalls of intercostal nerve blocks include difficulty in placement, high rate of local anesthetic absorption, and risk of pneumothorax. Ultrasound guidance has diminished these risks.
Intercostal Anatomy
The anatomy of the intercostal nerves and spaces is depicted in Figs. 12.1, 12.2, 12.7, and 12.8. Intercostal nerves are derived from the spinal roots of the respective thoracic segments. They are composed of dorsal horn sensory afferent fibers, ventral horn motor efferent fibers, and postganglionic sympathetic nerves that join the nerve via the paravertebral gray rami communicantes. Thus, each intercostal nerve has autonomic and somatic sensory and motor functions. Soon after the sympathetic contribution occurs within the paravertebral space, the intercostal nerve divides into ventral and dorsal rami. The dorsal ramus provides sensory innervation to the posteromedial structures of the back (synovium, periosteum, fascia, muscles, and skin) and motor innervation to the erector spinae muscles. The ventral ramus travels between the ribs. It is protected within the subcostal groove by the rib and two layers of intercostal muscle.
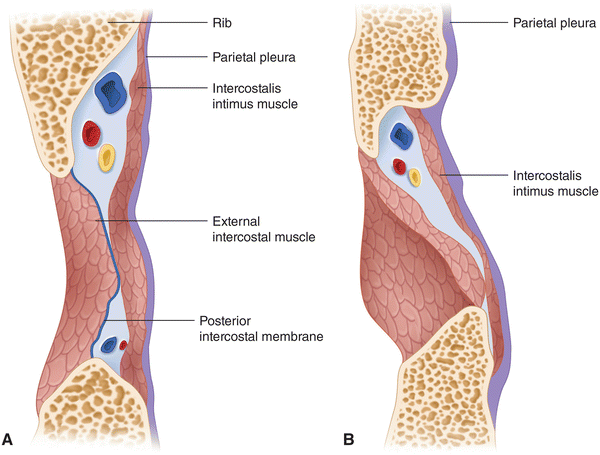
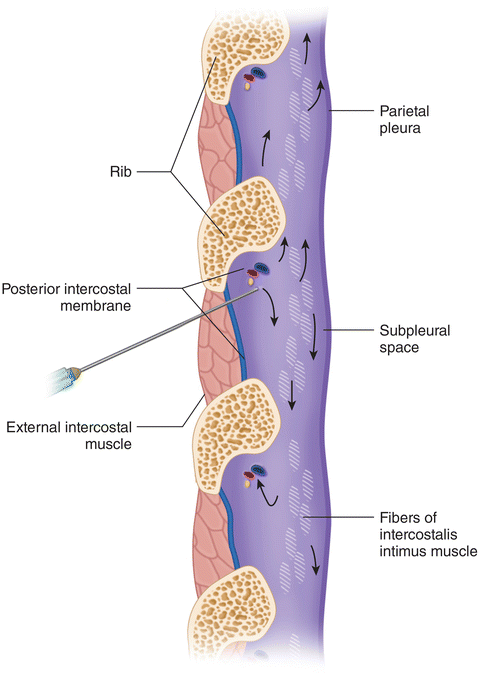

Fig. 12.7
Anatomic cross section through the intercostal space at (A) angle of Rib and (B) Laterally at the posterior axillary line (Reprinted from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)

Fig. 12.8
The aponeurosis or intercostalis intimus, does not impede spread of injectate to adjacent intercostal spaces when the needle or catheter is placed in the correct tissue plane (Modified from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)
Each intercostal nerve (ventral ramus) is associated with a vein and artery. The intercostal vein is derived from the confluence of venules along the thoracic cage and empties into the azygous vein on the right and the hemiazygous vein on the left. The most cephalad intercostal veins join and empty into the respective brachiocephalic veins bilaterally. The intercostal arteries are derived directly from the aorta.
The neurovascular structures are always superficial to the parietal pleura and thin aponeurotic-areolar tissue called the intercostalis intimus muscle. The aponeurotic-areolar tissue has muscle fibers embedded within its substance, and despite its name, its classification as a true muscle is a matter of debate among anatomists. There is various cutaneous branching of the ventral rami. In general, there are anterior and lateral branches, which divide and innervate skin and intercostal muscles of an individual segment along with variable collateral innervation of the adjacent segments. Because of this collateral innervation, it is necessary to block a level above and below the desired level. Because there is minimal adhesion of the aponeurosis to the parietal pleura, and the intercostalis intimus muscle is a rather flimsy structure, cephalad and caudad spread of injected solution to the adjacent intercostal spaces is not impeded (Fig. 12.7 and 12.8). It is important to keep in mind that the intercostal and paravertebral spaces are contiguous at all levels. Spread of local anesthetic to the paravertebral space produces unilateral segmental sympathetic blockade.
Intercostal Nerve Block Techniques
Intercostal neural blockade can be achieved intermittently or continuously in one or several segments depending on the technique used. Careful attention to technique decreases the rate of complication. Percutaneous injection of 2–5 mL of local anesthetic in at least three adjacent levels will ensure anesthesia/analgesia in the distribution of the middle intercostal nerve because of collateral innervation. Although relief is temporary, this technique is very effective in alleviating somatic pain in the chest wall and abdominal wall. Prolonged blockade requires either multiple reinsertions with the attendant risk of pneumothorax, placement of a catheter for bolus dosing or continuous infusion [80], injection with a neurolytic agent [81], or cryoablation [82].
Another important risk to keep in mind is local anesthetic toxicity. Blood levels of local anesthetic after intercostal blockade and interpleural analgesia are significantly greater than after any other frequently performed regional anesthetic techniques. Tucker et al. performed epidural, caudal, intercostal, brachial plexus, and sciatic/femoral nerve blocks with a single injection of mepivacaine 500 mg (1 % and 2 % solutions) with and without epinephrine [83]. When measuring arterial plasma levels, the highest levels were found after intercostal nerve blocks without epinephrine (5–10 μg/mL). When epinephrine was added to the solution (1:200,000 concentration), the plasma level decreased to 2–5 μg/mL. Epinephrine should be uniformly added to local anesthetic for performance of intercostal nerve block to minimize the potential for systemic toxicity.
Posterior Approach
Traditionally, intercostal nerve blocks are performed with a posterior approach at the angle of the rib, 6–8 cm lateral to the respective spinous process [84]. This target point allows direct palpation of the rib in most patients. It also allows blockade of the lateral intercostal cutaneous branch, which usually originates distal to the angle of the rib, ensuring good medial as well as lateral analgesia. The immediately adjacent intercostal nerves must also be blocked, because there is collateral innervation from the levels above and below. Neurolytic injections and cryoablative procedures must also be performed in a similar manner.
Figure 12.9 shows a technique for safely performing an intercostal nerve block. The skin above one intercostal space is retracted in a cephalad direction by the index and middle fingers of the nondominant hand. The rib corresponding to the nerve to be blocked is now between the fingers. A short-beveled, 25-gauge needle is advanced toward the inferior margin of the rib until bone is gently contacted. The fingers then release the skin to its original position. The needle is carefully walked off the inferior margin of the rib and advanced 3–5 mm, passing the external and internal intercostal muscles and placing the tip in the intercostal space. The width of the posterior intercostal space at the angle of the rib is approximately 8 mm [84]. Aspiration must be negative for blood and air. A volume of 2–5 mL of local anesthetic with 1:200,000 epinephrine is then slowly injected. This exact procedure is then repeated at the level above and below the targeted intercostal nerve. If multiple dermatomes need to be blocked, one level above and one below the targeted levels must also be blocked.
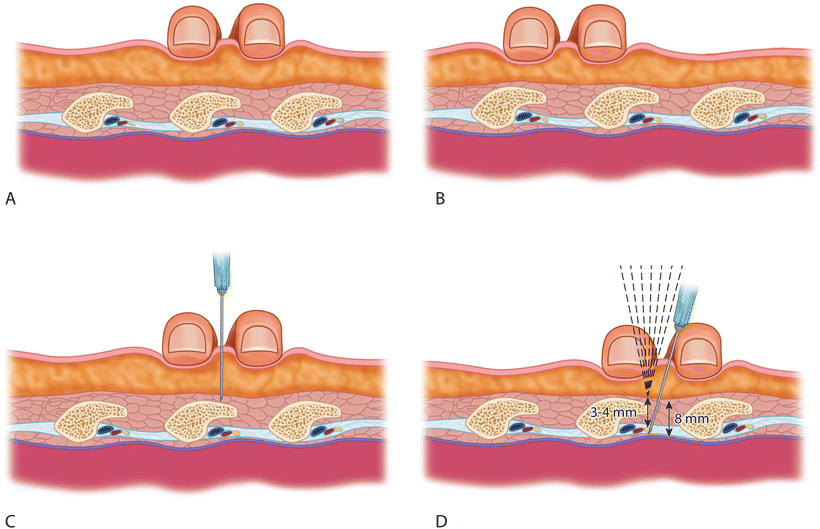

Fig. 12.9
Technique for intercostal nerve block. (a, b) The skin is retracted cephalad by two fingers straddling a rib. (c) A 25-gauge needle is advanced toward the inferior aspect of the rib until bone is contacted. (d) The cephalad traction on the skin is released, the needle is “walked off” the inferior border of the rib and advanced 3–5 mm beyond the rib to pass through the external and internal intercostal muscles (Modified from Ferrante FM, VadeBoncouer TR. Postoperative Pain Management. New York: Churchill Livingstone; 1993, with permission from Elsevier)
For pain associated with video-assisted thoracoscopy procedures, the utilization of intercostal nerve blockade with 0.375 % bupivacaine resulted in a significant decrease in the postoperative use of intravenous morphine [2]. This technique may be particularly useful for outpatient video-assisted thoracoscopy procedures.
Lateral Approach
A variation of this technique is entry at the posterior or midaxillary lines. These approaches may be adequate for blocking the anterior chest or abdominal wall, but will often miss the lateral cutaneous branch, thus providing less than satisfactory blockade of the back and flank regions.
In patients undergoing thoracotomy, the surgeon may perform the blocks under direct visualization just before closure. However, these blocks are often placed at a site more medial than what would be chosen for a percutaneous approach. Thus, there seems to be a higher incidence of complications because of the proximity to the spinal nerve roots.
Continuous Technique
Nunn and Slavin described the ability of a single intercostal injection of India ink to spread subpleurally to multiple intercostal spaces [84]. The minimally adherent parietal pleura and the thin intercostalis intimus muscle did not hinder the multidirectional spread of the injectate (Fig. 12.8).
Based on morphometric measurements of the intercostal space, Nunn and Slavin placed the needle tip 3 mm past the inferior margin of the rib, leaving approximately 5 mm to the pleura. In a study by O’Kelly and Garry, a continuous catheter was placed through a 19-gauge epidural needle with the tip directed medially [85]. After first injecting 10 mL of solution through the needle, the catheter was advanced 2 cm and then secured to the skin. Appropriate spread of local anesthetic was confirmed by radiographic imaging.
Satisfactory analgesia has been documented using continuous infusion [86]. Seventy-five patients (92 %) had good analgesia without requiring supplemental medications during the first postoperative day using an infusion of 0.5 % bupivacaine at 7 mL/h. Sixty-six patients (81.5 %) remained satisfied with their analgesia over the following 4 days. Patients who experienced inadequate analgesia early in their course were thought to have leakage of anesthetic into the interpleural space. Subsequent decrements in analgesic efficacy were attributed to tachyphylaxis. The same authors modified the protocol to increase the infusion rate to a maximum of 10 mL/h [87]. This resulted in a significant improvement in pulmonary function over the control group, which required higher doses of intravenous rescue pain medications than the continuous intercostal infusion group.
Ultrasound-Guided Intercostal Nerve Block
The rib at the level to be blocked is by palpation and traced posterior to the posterior angulation of the affected rib. A linear high-frequency ultrasound transducer is then placed in the longitudinal plane with the superior aspect of the ultrasound transducer rotated about 15° laterally over the affected rib at the posterior angulation of the ribs. The rib can be seen as a hyperechoic curvilinear line with an acoustic shadow underneath it. The three layers of intercostal muscle, the external, internal, and innermost, are identified in the intercostal space between adjacent ribs. Color Doppler helps identify beneath the adjacent intercostal artery and vein. This space between adjacent ribs provides an excellent acoustic window, which allows easy identification of the intercostal space and the pleura beneath it. The depth of the pleura is noted. Usually, both the rib inferior to the targeted rib and the targeted rib can be visualized in the same window. 22-gauge echogenic needle is advanced from the inferior border of the ultrasound transducer using an in-plane approach with the trajectory being adjusted under real-time ultrasound guidance until the needle tip is resting in the internal layer of the intercostal muscle. At that point, after careful aspiration, a small amount of solution is injected under real-time ultrasound imaging to utilize hydrodissection to reconfirm the position of the needle tip. Once the position of the needle tip is reconfirmed, the needle is carefully advanced into the innermost layer of the intercostal muscle just short of the previously identified depth of the pleura. After careful aspiration, a small amount of solution is again injected to aid in identification of the position of the needle tip with attention paid to the relative location of the bright hyperechoic pleural line. After careful aspiration, the remainder of the solution is slowly injected. There should be minimal resistance to injection. The needle is then removed and a sterile dressing is applied at the injection site [88].
Complications/Treatment
The most common complications of intercostal nerve block are associated with the aberrant needle placement (pneumothorax, hemothorax, hemoptysis, hematoma, intravascular injection, neuritis, subarachnoid block, failed block) or problems associated with the injectate (allergic reaction, toxic reaction, epinephrine reaction, tissue necrosis, respiratory insufficiency).
The actual incidence of pneumothorax secondary to intercostal nerve block is quite small. A large, retrospective study reporting 50,097 intercostal nerve blocks in 4333 patients undergoing surgery or therapeutic nerve blocks revealed only four clinically significant pneumothoraces (0.092 %) and no other significant complications [89]. The technique for intercostal neural blockade was similar to the posterior approach described by Nunn and Slavin [84]. There was some minor discomfort at the injection sites in 5 % of patients. A prospective study by the same authors in 200 consecutive patients undergoing intercostal nerve block compared pre- and postinjection films to evaluate for pneumothorax [90]. There were only four pneumothoraces in a total of 2610 needle punctures, of which three pneumothoraces were attributed to the actual surgical procedure itself and not performance of the blocks. In the largest retrospective study with more than 100,000 needle punctures, Moore reported an incidence of pneumothorax of 0.073 % without any other serious complications [90]. It is important to note that residents still in training performed most of these blocks [91].
There are sporadic case reports of other types of complications. Hematoma has occurred in a heparinized patient [92]. Bilateral intercostal nerve blocks have resulted in postoperative respiratory failure in patients with preoperative pulmonary compromise [93, 94]. Motor blockade and the loss of accessory respiratory muscle function were the hypothesized etiologic mechanisms. In a study looking at the efficacy of continuous epidural versus intercostal analgesia, one intercostal catheter led to rib osteomyelitis which had to be treated surgically [80]. Local anesthetic toxicity can occur due to higher absorption due to the close proximity of the intercostal vasculature.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





