Fig. 13.1
12-lead ECG
The initial physical exam was notable for an anxious and diaphoretic patient with elevated jugular venous pressure, crackles at the lung bases, and a left ventricular S3 with a soft systolic murmur adjacent to the lower left sternal boarder. Initial blood tests showed WBC 12.5, hemoglobin 15.5 mg/dL, serum creatinine 1.2 mg/dl, and cardiac troponin I 6.06 ug/L. She was brought emergently to the cardiac catheterization laboratory where she was found to have 100 % occlusion of a dominant, mid-left circumflex artery. She underwent successful PCI with placement of two overlapping drug-eluting stents, and restoration of TIMI 3 flow to the infarct related artery (Table 13.1). At the conclusion of the case, the patient became agitated and pulse oximetry showed 88 % saturation despite administering 100 % oxygen by non-rebreather facemask. Blood-pressure was 78/58 mmHg at this time, and the patient required endotracheal intubation and the initiation of vasopressor medications to stabilize her blood pressure.
Table 13.1
TIMI flow definitions
TIMI Grade 0 (no perfusion) | There is no antegrade flow beyond the point of occlusion |
TIMI Grade 1 (penetration without perfusion) | The contrast material passes beyond the area of obstruction but “hangs up” and fails to opacify the entire coronary bed distal to the obstruction for the duration of the cineangiographic filming sequence |
TIMI Grade 2 (partial perfusion) | The contrast material passes across the obstruction and opacifies the coronary bed distal to the obstruction. However, the rate of entry of contrast material into the vessel distal to the obstruction or its rate of clearance from the distal bed (or both) are perceptibly slower than its entry into or clearance from comparable areas not perfused by the previously occluded vessel – e.g., the opposite coronary artery or the coronary bed proximal to the obstruction |
TIMI Grade 3 (complete perfusion) | Antegrade flow into the bed distal to the obstruction occurs as promptly as antegrade flow into the bed proximal to the obstruction, and clearance of contrast material from the involved bed as rapid as clearance from an uninvolved bed in the same vessel or the opposite artery |
Question
What is the differential diagnosis for the patient’s hemodynamic and respiratory decompensation, and what should be done next to confirm the diagnosis?
Answer
The differential diagnosis must include mechanical complications of AMI including ventricular septal rupture (VSR) (Video 13.1), papillary muscle rupture leading to acute mitral regurgitation (MR) (Video 13.2), or free-wall rupture leading to pseudoaneurysm or cardiac tamponade (Video 13.3). Other etiologies to be considered include RV infarction, acute blood loss, iatrogenic hypotension secondary to medication, and cath lab complications such as aortic dissection or coronary perforation. The most rapid way to confirm the diagnosis is with immediate, trans-thoracic echocardiography.
The patient has demonstrated hemodynamic instability despite patency of the infarct related artery in the setting of a delayed presentation of acute myocardial infarction (AMI). Based on her clinical and hemodynamic findings she is now Killip Class IV (Table 13.2). Understanding the etiology of her hemodynamic instability is now crucial to guide further management. While this may represent LV failure in the setting of a large MI, it is unusual that an isolated infarction in the distribution described will account for these findings. Mechanical complications of MI, as listed above, must be considered and rapidly identified or excluded. In this specific case, the bedside echo demonstrated a severe, eccentric jet of mitral regurgitation. On a subsequent trans-esophageal echocardiogram (TEE), the diagnosis was confirmed to be due to rupture and flail of the papillary muscle with prolapse into the left atrium during systole. An image from the trans-esophageal echocardiogram obtained in the standard 4-chamber view is provided in Fig. 13.2. The patient was taken emergently to the operating room where a bioprosthetic mitral valve replacement was performed. The patient was successfully discharged to a sub-acute nursing facility on hospital day 15, and made a full recovery.
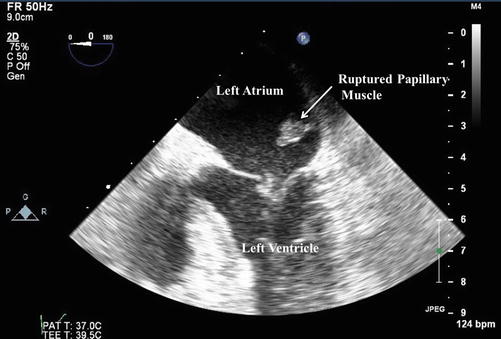
Table 13.2
Killip Class definitions
Definition | 30-day mortality (in 1967) (%) | |
|---|---|---|
Killip Class I | No clinical signs of heart failure | <6 |
Killip Class II | Signs of heart failure including rhales or crackles in the lower lung fields, an S3, or elevated jugular venous pressure | <17 |
Killip Class III | Frank, acute pulmonary edema. | 38 |
Killip Class IV | Cardiogenic shock or hypotension (measured as systolic blood pressure < 90 mmHg) and evidence of peripheral vasoconstriction (oliguria, cyanosis, or sweating) | 81 |

Fig. 13.2
Trans-esophageal echocardiogram
Standard Approach to Management
Epidemiology
Cardiogenic shock in the peri-infarct setting is defined as inadequate tissue perfusion and sustained hypotension despite adequate intravascular volume. Cardiogenic shock complicates between 5 and 8 % of cases of AMI in contemporary databases and trials. Mortality for AMI in general is <5 % in contemporary series, but can exceed 50 % in patients with shock [1]. The most common cause of shock after AMI is left ventricular (LV) failure which represents 80 % of cases, but mechanical complications of AMI must be considered including isolated right ventricular (RV) infarct, ventricular septal rupture (VSR), free-wall rupture with ensuing cardiac tamponade, and papillary muscle rupture leading to acute mitral regurgitation (MR). Data from the SHOCK trial and the subsequent SHOCK registry documented the most common etiologies of cardiogenic shock among 1,422 patients after AMI (Fig. 13.3) [2].
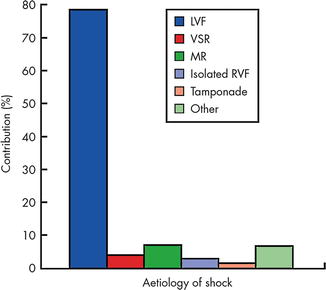

Fig. 13.3
Etiology of suspected cardiogenic shock in the combined SHOCK trial registry and trial (total n = 1422, only first 232 trial patients are included). “Other” includes shock caused by prior severe valvular disease, dilated cardiomyopathy, excess beta-blockade/calcium channel blockade, hemorrhage, and procedural complications. Aortic dissection, pulmonary embolism, and dynamic subaortic outflow obstruction should also be considered. LVF left ventricular failure, MR mitral regurgitation, RVF right ventricular failure, VSR ventricular septal rupture (Reproduced with permission from Menon, Heart, 2002 [2] with permission from BMJ Publishing Group Ltd)
Diagnosis
The first step in managing patients with cardiogenic shock after AMI is to identify the cause. Specifically, one must attempt to differentiate between mechanical complications that require immediate operative treatment as compared to LV failure alone. A rapid but thorough physical examination is important in any patient with AMI and shock. Cardiac auscultation may reveal the characteristic, harsh, holosystolic murmur of a VSR or a softer, apical murmur consistent with acute mitral regurgitation. Cardiac tamponade may be suspected based on jugular venous distention (JVD) and a pulsus paradoxus, or Beck’s triad of hypotension, distended neck veins, and muffled heart sounds. Patients with RV infarction may display a pulsus paradoxus along with the Kussmaul sign (increase in JVD with inspiration) which is usually absent in patients with cardiac tamponade. Physical exam may be challenging due to the degree of hypotension, the need for mechanical ventilation, and the physical environment. The murmur of acute MR may be soft and difficult to appreciate due to rapid elevation in left atrial pressures. Similarly a large VSR in the setting of hypotension may also be under appreciated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







