 Box 14.1 Common Causes of Sudden Collapse with Coma
Box 14.1 Common Causes of Sudden Collapse with Coma- Subarachnoid haemorrhage
- Brain-stem cerebral vascular accident (CVA)
- Hypoglycaemia
- Poisoning (e.g. tricyclic antidepressants)
- Head injury
E – Environment and Exposure
- Take the temperature (tympanic or rectal).
- Remove clothing (but keep warm), and examine the whole patient – front and back. Search the clothing for treatment cards, drugs, suicide notes and identification. Consider forensic needs.
- Protect the eyes.
- Consider specific treatment for:
- hypothermia → p. 243
- infection
- hyperpyrexia → pp. 245 and 271.
- hypothermia → p. 243
F – Fits
- Control any tonic–clonic activity with benzodiazepines (→ p. 231).
- Protect the tongue from the teeth with a Guedel airway.
G – Glucose
Check the blood sugar in any patient who is unresponsive or behaving strangely and administer IV glucose if necessary.
H – History
- Get as much detail as possible from bystanders, ambulance crew and relatives. The essentials are AMPLE (→ p. 12).
- Obtain hospital records or details from the GP by telephone.
I – Immediate Analgesia and Investigations
- Give analgesia, if required, and look for the cause of any pain.
- Request a chest radiograph (CXR).
- Obtain an ECG.
- Take blood for full blood count (FBC), urea and electrolytes (U&Es), sugar and C-reactive protein (CRP). Consider also requesting a serum osmolality. The osmolar gap is a useful and easily available indicator of toxic and metabolic disorders → Boxes 14.2 and 14.3.
- Consider the need for a nasogastric tube and/or a urinary catheter in all unresponsive patients.
- Consider the need for radiographs of the hips and pelvis in any older person who has collapsed or been found on the floor.
 Box 14.2 The Osmolar Gap
Box 14.2 The Osmolar Gap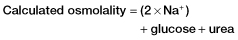
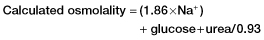
- ethanol
- methanol
- ethylene glycol
- mannitol.
 Box 14.3 The Anion Gap
Box 14.3 The Anion Gap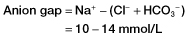
Poisoning
Unexplained collapse with generalised or bizarre signs must always raise the possibility of poisoning, even if this is strenuously denied. In unresponsive patients, a gastric lavage may yield useful information and remove toxic substances from the stomach.
Collapse in Children
Collapse in children usually accompanies overwhelming pathology such as shock, respiratory failure or intracranial infection.
Collapse in Elderly People
Collapse in elderly people is common because of the following:
The cause of a sudden deterioration in an elderly patient may not be immediately apparent from the history or clinical examination. For this reason, most collapsed older patients will need investigation before discharge to exclude diagnoses such as the following:
- MI or pulmonary embolism
- Heart block or other dysrhythmia
- Pneumonia, pyelonephritis or other infection
- Gastrointestinal bleeding or other blood loss
- Anaemia or renal failure
- Chronic subdural haemorrhage or CVA
- Fractured neck of femur.
FBC, standard blood chemistry, glucose, ECG and CXR are essential. CRP, plasma calcium and osmolality are useful in non-specific illness.
Patients who have been found lying on the floor are often cold, dehydrated and frightened; they must be admitted for further assessment and stabilisation. The plasma creatinine kinase should be measured and the urine tested for myoglobin (For crush injuries → p. 265 and hypothermia → p. 243).
Prevention of Pressure Sores
Pressure sores cause a great deal of morbidity among hospitalised patients and are difficult and expensive to treat. They will begin to form or worsen during a period of immobility on an emergency department (ED) trolley, especially if the time is >2 h. Thus all patients who are recumbent should be assessed for risk factors using a predetermined scoring system.
There are general risk factors including the following:
- Obesity or low body weight
- Thin, oedematous, sweaty or damaged skin
- Reduced or restricted mobility, including that caused by pain or splintage
- Incontinence of urine or faeces
- Low intake of food and drink
- Increasing age (>50 years)
- Female gender.
Problems that carry a special and very high risk of skin breakdown include the following:
- Cachexia
- Cardiovascular disease
- Neurological disease
- Major trauma
- History of lying on the floor
- Hypothermia
- Treatment with steroids and cytotoxics.
Patients who are found to be at risk of developing pressure sores should receive pressure area care from trained nursing staff and be moved to a suitable ward bed as soon as possible.
Prevention of Venous Thromboembolism
Venous thromboembolism (VTE) usually starts as a deep vein thrombosis (DVT) and is not just a problem in surgical patients. Sick patients of all types are susceptible, especially if their mobility is reduced for 3 days or more. Moreover, the risk of DVT doubles with serious infections. Up to 20% of general medical patients are believed to have VTE that is very often undiagnosed. The incidence of VTE has been found to be 100 times greater in hospital patients than in similar populations in the community. It is the most common cause of preventable death in British hospitals and is responsible for 25 times more deaths than meticillin-resistant Staphylococcus aureus (MRSA)! For this reason, prophylaxis against DVT is recommended for almost all medical patients. Exceptions include those who present with a condition that might be haemorrhagic in origin or who:
- are aged <40 years and fully mobile
- have a congenital or acquired bleeding disorder (including severe liver disease)
- have thrombocytopenia (platelets <75 × 109/L)
- are already on anticoagulants (with an international normalised ratio or INR >2)
- have a history of haemorrhagic stroke or subarachnoid haemorrhage
- have uncontrolled systolic hypertension (>230/120 mmHg)
- have a recent history of GI bleeding
- are known to have pericarditis or diabetic retinopathy
- have had a lumbar puncture or epidural injection in the previous 4 h or are expected to require one of these procedures in the next 12 h.
A suitable regimen is enoxaparin 40 mg daily by subcutaneous injection, started in the ED. Alternative anticoagulants for this purpose include other low-molecular-weight heparins, unfractionated heparin and fondaparinux. Mechanical DVT prophylaxis with stockings or pneumatic devices is also possible. Aspirin and other antiplatelet agents do not provide adequate protection against VTE.
Driving After a Collapse or Sudden Illness
Patients who have conditions that may result in a sudden and dramatic change in health are not fit to drive or operate dangerous machinery → p. 376. An escort home should be arranged for all such patients and they should be warned of the possible dangers to themselves and others. For advice on driving after a CVA or transient ischaemic attack (TIA) → p. 230.
NEUROLOGICAL PROBLEMS
Syncope (Transient loss of Consciousness)
Transient loss of consciousness (‘blackout’) is a common presentation to an ED, particularly in teenagers, young adults and elderly people. It affects almost half the population at some point in their lives. A careful history is essential to ascertain the following:
- What the patient was doing at the time of the syncope
- What posture he or she was in
- What warning signs were experienced
- Whether the person feels that he or she has fully recovered
- The past medical history and medications.
‘Red flag’ symptoms include:
- Collapse and loss of consciousness during exertion
- Dyspnoea, chest pain or headache
- Tongue-biting, incontinence or post-ictal drowsiness/confusion
- Family history of sudden cardiac death before age 40.
Information from observers and paramedics can be extremely useful in this respect.
Subsequent examination and investigations can be tailored to the likely causes of the syncope as suggested by the history. Older people will require an ECG and possibly routine blood tests and measurement of lying and standing BPs. Possible diagnoses at all ages include epilepsy, cardiac causes and psychogenic syncope.
Vasovagal syncope
(the common faint) often occurs in pregnancy or during the prodromal phase of a viral illness. There should be a history of some warning symptoms before the faint, a full recovery and no features to suggest alternative diagnoses. A mild degree of bradycardia is a common finding even when the patient appears to have fully recovered. Sudden arising to an upright posture, prolonged standing, excessive heat and anticipated medical procedures may all induce this type of syncope. Occasionally, a vasovagal faint will be followed by a brief convulsion – an ‘anoxic’ fit. The patient can be reassured if there is a good history of a simple faint, no previous similar episodes, and a full and rapid recovery.
Situational syncope
may occur during micturition or coughing. There should be a clear history, a full recovery and no features to suggest alternative diagnoses.
Orthostatic syncope
is caused by postural hypotension and can be diagnosed when there is a drop in systolic blood pressure upon standing of >20 mmHg (or a drop in diastolic BP >10 mmHg). The first BP reading should be taken after the patient has been lying supine for 5 min and then the second reading is taken 1 min later after standing. Postural hypotension is usually associated with medications such as vasodilators, antihypertensives and antidepressants. Dehydration, arteriosclerosis, diabetes and some other metabolic diseases are also implicated in orthostatic drops in BP. A change of drug therapy should be considered in liaison with the GP.
Some patients with transient loss of consciousness can be safely discharged whereas others will require admission. Those who have fully recovered but have some worrying features, a history of a possible fit or abnormal investigations should be sent to an appropriate specialist clinic. Patients aged >65 years with no prodromal symptoms should always be considered for further investigation. All patients who are waiting for specialist assessment should be warned not to drive and to take appropriate precautions at home and at work.
Headache
The discomfort described as a headache may result from the following:
- Muscular tension (tension headache)
- Arterial dilatation (hypertension, nitrates)
- Traction on arteries (raised intracranial pressure)
- Traction on venous sinuses (low pressure headache after cerebrospinal fluid leak or dehydration)
- Inflammation (meningitis, arteritis)
- Referred pain (sinusitis, glaucoma).
 Box 14.4 Causes of a Headache
Box 14.4 Causes of a Headache- Tension headache
- Migraine/cluster headache
- Subarachnoid haemorrhage (SAH)
- Other intracranial bleed
- Post head injury headache
- Intracranial infection
- Systemic febrile illnesses
- Space-occupying lesion
- Temporal arteritis
- Malignant hypertension
- Paget’s disease
- Hypercapnia/hypoxia
- Poisoning (CO2, nitrates)
- Glaucoma
- ENT infection and nasal trauma
- Dental problems
- Neck problems
- Cerebrospinal (CSF) fluid leakage
- Dehydration
- Psychogenic factors, including anxiety and depression
Worrying features in the history of a patient with a headache include:
- sudden onset of symptoms
- neck stiffness
- loss of consciousness, however transient
- fit or other collapse
- visual loss or other visual change
- other neurological symptoms
- change in personality, memory or mental ability
- pain worse on waking or straining
- worst-ever headache
- first severe headache in a patient aged >35 years.
The minimum examination of an ambulant patient with a headache should record the following:
- Cortical functioning/Glasgow Coma Scale (GCS)
- Gross neurological state
- Pupillary signs
- Fundoscopy
- The presence or absence of meningism or neck tenderness
- Temperature
- The presence or absence of rashes
- Otoscopy
- BP
- Palpation of the scalp and sinuses for tenderness.
XR
CT is the most useful first-line investigation of a headache unless lumbar puncture is needed to exclude meningitis. A negative scan does not rule out SAH.
TX
Patients with a headache who have symptoms or signs suggestive of serious pathology should be referred to the physician on call. Even with no neurological signs and a GCS score of 15/15, more than 1 in 20 patients with a ‘thunderclap’ headache will be found to have an SAH.
- 50% of patients with SAH have a warning headache in the week before a big bleed
- 50% have a warning headache without major physical signs
- nearly 50% have seen a doctor about the headache
- 50% with missed initial bleeds die
Head Injury
Head injury must always be considered as a cause of collapse and may often coexist with it. Where there is doubt as to the relative influences of intracranial injury and other conditions, then a CT scan should be performed.
Subarachnoid Haemorrhage
Primary SAH occurs in 1 in 1000 adults. Most (80–85%) of these bleeds are caused by the rupture of saccular (‘berry’) aneurysms, usually situated around the circle of Willis or one of its major branches. In the remaining 15–20% of cases, no aneurysm is detected and about half of these bleeds are attributed to non-aneurysmal perimesencephalic haemorrhage, in which the blood is limited to the subarachnoid spaces around the midbrain (i.e. the mesencephalon) and its exact origin is uncertain. Another group of spontaneous SAHs are due to other disorders affecting the blood vessels (such as arteriovenous malformations). If untreated, saccular aneurysms have a 20–40% chance of re-bleeding in the following month with an 80% chance of death or residual disability. Perimesencephalic haemorrhages have a very good prognosis and can be treated conservatively. Overall, 10% of patients with primary SAH die in the first few hours and this rises to 50% in the first few weeks. Bleeding recurs in one in five patients, usually at the end of the first week. Secondary SAH may occur from extension of an intracerebral bleed or after trauma.
SAH usually presents as one of the following:
Meningism is often present even in the comatose patient although neck pain and photophobia are not essential for the diagnosis. Lateralising signs are usually ‘soft’ or absent.
Fundoscopy of comatose patients with SAH may reveal preretinal (subhyaloid) haemorrhages; this sign is pathognomonic. Tropicamide mydriatic drops may be used to dilate the pupils; pupillary signs must be recorded first.
Investigations:
Non-contrast CT scan of the brain is more than 95% sensitive at detecting subarachnoid blood. It also shows the amount and distribution of the blood and complications such as surrounding oedema, hydrocephalus or ischaemic damage. Larger bleeds or those with extension into the brain parenchyma almost always yield a positive result. However, the sensitivity of CT falls to less than 50% after a week and the blood may have been completely reabsorbed by 10 days.
When the history is suggestive of SAH and the CT scan is negative, lumbar puncture will be required. This should not be performed until at least 6 h (and preferably 12 h) have elapsed since the onset of symptoms. A bloody tap can be distinguished from true SAH by levels of red cells that remain constant in successive collection tubes and by the presence of breakdown products of haemoglobin. MRI, conventional catheter angiography and MR or CT with angiography are all used as further investigations for SAH.
TX
- ABCs
- Terminate convulsions
- Check the blood sugar
- Refer the patient to the general physicians/neurosurgeons as appropriate.
The calcium antagonist nimodipine (60 mg every 4 h) is used to prevent the development of secondary cerebral ischaemia. This occurs in 50% of patients, usually in the first 5–10 days after the bleed.
Intracranial Infection
Impaired consciousness and pyrexia suggest intracranial infection. Meningitis is the most common cause and may be associated with infection elsewhere. Encephalitis (usually herpetic) may present as confusion, disorientation, drowsiness or bizarre behaviour.
Meningococcal disease is especially important for ED personnel to recognise because very early treatment (with IV antibiotics, fluids and ventilation) improves the prognosis. In the UK, cases of meningococcal infection, both meningitic and septicaemic, usually rise in the autumn and reach a peak early in the New Year. This annual rise in cases starts in students in colleges of higher education, although the highest incidence rates are in children aged <2 years. Muslim visitors to Saudi Arabia for the Hajj or Umrah pilgrimages are also at a high risk of meningococcal infection.
Temporal Arteritis
Cranial or giant cell arteritis is a panarteritis of unknown aetiology, which affects medium-sized vessels, particularly those of the scalp. Affected arteries are thickened and tender and thrombosis may occur. The condition is related to polymyalgia rheumatica and is predominantly seen in patients aged >60 years (and hardly ever in people aged <50). The incidence of giant cell arteritis continues to rise with increasing age.
Temporal arteritis – the most common manifestation – starts with an acute onset of headache. There is a sharp, burning pain over the temporal artery, which may be locally tender or even pulseless. Neurological signs are caused by ischaemia in the territory of other inflamed arteries:
- Visual impairment: which may progress to blindness caused by ischaemia of the optic nerve and retina
- Ophthalmoplegia: due to ischaemia of the ocular motor nerves
- Pain on eating: due to masseter claudication
- TIA/stroke: caused by involvement of branches of the vertebral or carotid arteries.
Systemic malaise and weight loss may sometimes occur. In many cases, the features are atypical.
Investigations:
A raised erythrocyte sedimentation rate (ESR) may confirm the diagnosis but a normal ESR does not exclude it. Plasma CRP is also elevated in most cases. Serial ESRs and biopsy of the superficial temporal artery may be required.
TX
As soon as the diagnosis is suspected, parenteral methylprednisolone should be given (up to 500 mg by slow IV injection). Oral prednisolone (40 mg) is an alternative (60 mg if there are visual symptoms). Soluble aspirin (300 mg) should also be administered. Prompt treatment with high-dose systemic steroids does not usually improve vision but prevents further deterioration, especially in an eye that has not already been affected. Analgesia and admission for observation are also required. Recovery can take several months and recurrence is always a possibility.
Migraine
The first severe attack of migraine usually occurs in the teenage years or early 20s. There may be an isolated unilateral throbbing headache (common migraine) or a more complicated syndrome preceded by some form of aura (classic migraine). In the latter case, the headache may be accompanied by:
- gross malaise
- pallor and tremor
- nausea and vomiting with gastric stasis
- visual disturbance
- abdominal pain
- focal neurological signs such as paraesthesia and numbness or even hemiparesis.
Over half of all patients with migraine have a family history of the condition and many have suffered from travel sickness as children.
Episodes of migraine may last for hours or even days. Those attacks that are atypical in severity or duration should be given special consideration.
TX
A severe headache can often be avoided if simple analgesics are taken early enough in the course of an attack. Unfortunately, by the time most patients present to an ED, the opportunity for this has passed and vomiting then makes oral drugs useless. A parenteral non-steroidal anti-inflammatory drug (NSAID) (e.g. diclofenac 75 mg IM) can be given together with an antiemetic injection. Sumatriptan is a specific treatment for migraine (→ Boxes 14.5 and 14.6) but it is only effective in about 75% of patients.
 Box 14.5 Use of Sumatriptan
Box 14.5 Use of Sumatriptan- lethargy
- dizziness or vertigo
- sensations of warmth
- feelings of pressure or heaviness in any part of the body
 Box 14.6 Contraindications to Sumatriptan
Box 14.6 Contraindications to Sumatriptan- Known hypersensitivity to the drug
- Ischaemic heart disease
- Conditions predisposing to ischaemic heart disease
- Prinzmetal’s angina
- Poorly controlled hypertension
- Renal or hepatic impairment
- Hemiplegic migraine
- Patients taking monoamine oxidase inhibitors, selective serotonin reuptake inhibitors (SSRIs) or lithium
- Pregnancy or lactation
- Children
- Age >65 years
Neurological symptoms should be treated by oral aspirin and admission for observation. Most severe attacks finally terminate when the sufferer falls asleep. Not surprisingly, a feeling of euphoria may accompany the subsequent relief.
Cluster Headache (Migrainous Neuralgia)
This is a related condition that is more common in men and is often misdiagnosed. There is severe unilateral facial pain (orbital, supraorbital or temporal) with lacrimation and rhinorrhoea. Each attack starts abruptly and lasts from 15 min up to 3 hours. In over 80% of patients, the headaches ‘cluster’ into episodes over a period of a few weeks before there is a time of remission. This unpleasant neuralgic headache is treated in a similar way to migraine in the acute phase although specialist assessment should be arranged.
Cluster headache is a member of a group of comparatively rare conditions called trigeminal autonomic cephalalgias. They are characterised by severe unilateral pain in a trigeminal distribution and are associated with ipsilateral cranial autonomic features. Paroxysmal hemicrania is another member of the group with similar features to cluster headache although attacks are shorter, more frequent and more common in women.
Tension Headache
This is the most common cause of a headache, occurring more often in females than in males. Symptoms may persist for several days and occur up to 14 days/month. The pain is usually described as a tight cap or band pressing around the head. The distribution of the sensation is that of the occipitofrontalis muscle and tension in this muscle is probably the cause of the pain. Nausea and difficulty getting to sleep may also be experienced. The muscular tension may be precipitated and maintained by:
- personal worries and difficulties
- constant unremitting stress (e.g. mother with young children)
- depression
- a minor injury (e.g. whiplash type).
TX
The patient has often found that simple analgesics have not relieved the pain. Non-steroidal anti-inflammatory drugs (NSAIDs) may help, as may psycho- and physiotherapy. Tricyclic antidepressants are sometimes prescribed in relatively low doses (e.g. amitriptyline 50–100 mg at night). Referral should be made to the GP. SAH must be excluded → p. 224.
Other Headaches:
Most other patients with headaches should also be referred back to the GP but those with neurological signs should be admitted for investigation.
Brain-Stem CVA
Sudden catastrophic collapse may be caused by haemorrhage into or infarction of the brain stem. There will be:
- coma
- small fixed pupils (exclude narcotic/opiate poisoning)
- few, if any, lateralising signs.
Respiration continues but other brain-stem functions and reflexes are attenuated. The prognosis is grave.
XR
It is important to make an accurate diagnosis as soon as possible to avoid inappropriate and distressing life support measures. A CT scan will be needed for this purpose.
TX
As soon as the prognosis is assured, the relatives must be gently informed. The patient should be admitted to a bed and kept comfortable while nature takes its course.
Cerebrovascular Events
Cerebrovascular disease is the third most common cause of death in the UK and the single most common cause of severe disability. After a stroke, approximately 23% of people die within 30 days and, of the initial survivors, only 30–40% are alive after 3 years and 25–30% remain permanently disabled. In Europe, people of West Indian origin have a particularly high risk of CVA.
Most CVAs (80%) are a result of thrombotic or embolic cerebral infarction rather than haemorrhage. The most common causes of cardiac emboli are atrial fibrillation (AF), mural thrombosis and valvular heart disease, and these emboli lodge in the large cerebral arteries, particularly the middle cerebral arteries. Atherosclerosis causes both intracerebral thrombosis and thromboembolism from the extracranial internal carotid arteries. It also affects the vertebral and basilar arteries. The Oxford system of stroke classification defines a total anterior circulation stroke as having three components: contralateral motor or sensory deficit, homonymous hemianopia and higher cortical dysfunction such as dysphasia and visuospatial disturbance. The combination of any two of these features is called a partial anterior circulation stroke. A posterior circulation stroke may manifest itself as cerebellar ataxia or brain-stem signs or isolated homonymous hemianopia. Occlusion of deep perforating arteries (which arise from both the anterior and posterior circulation and supply the white matter of the cerebral hemispheres and brain stem) causes a lacunar stroke. This leads to pure motor, pure sensory or mixed sensorimotor deficit.
Cerebral infarction leads to a progressive neurological deficit. Signs may be minimal at first with subjective weakness or paraesthesia only. The CVAs that result from intracerebral haemorrhage (the remaining 20%) are more sudden in onset with headache and rapidly established signs.
A stroke causes loss or disturbance of motor and sensory function. The exact clinical picture depends on the territory of the blood vessel involved (→ Box 14.7). Posterior lesions may affect conscious level (→ p. 227) or more rarely there may be cerebellar signs. In the very early stages, spinal reflexes are usually depressed although the plantar response may be extensor. Severe hypertension may be present but intervention to lower it in the ED is contraindicated because this may precipitate further cerebrovascular damage.
 Box 14.7 Symptoms of a TIA
Box 14.7 Symptoms of a TIA- Unilateral paresis
- Unilateral sensory loss
- Aphasia
- Monocular visual loss
- Bilateral or alternating weakness or sensory loss
- Bilateral visual loss/hemianopia
- Two or more of the following: vertigo, diplopia, dysphagia, dysarthria, ataxia
Investigations:
Routine blood tests, CXR and ECG should be performed. Coincidental hip fracture must be considered and excluded. Brain imaging should be undertaken as soon as possible in all patients, certainly within 24 h of the onset of symptoms unless there are good clinical reasons for not doing so. The brain scan must be performed urgently if the patient has:
- a known bleeding tendency or is taking anticoagulants
- a depressed level of consciousness
- a severe headache at the onset
- an uncertain diagnosis or atypical features (most doctors would consider age <60 years as atypical)
- unexplained, progressive or fluctuating signs
- papilloedema, neck stiffness or fever
- indications for thrombolysis or early anticoagulation (→ later).
Elderly people often fall at the onset of a stroke, but this history may be lacking. Traumatic intracranial blood (acute or chronic subdural or even extradural) may mimic a CVA. Sudden deterioration, in particular with a fixed, dilated pupil, raises the suspicion of an expanding lesion. A CT scan is indicated to confirm the suspected sequence of events.
TX
- Give oxygen, if required, to maintain the SaO2 >94%.
- Monitor vital signs. Current guidelines do not recommend routine lowering of BP in the acute phase of a stroke unless there are specific circumstances such as hypertensive encephalopathy or aortic dissection.
- Check the blood sugar. In the absence of diabetes, there is no evidence to support treating hyperglycaemia in the acute phase of a stroke.
- Establish IV access and give maintenance IV fluids. The patient should be kept nil by mouth until there has been a proper assessment of the airway and swallowing reflexes.
- Treat complications (e.g. fits).
- Treat pyrexia with standard drugs. It is associated with a poor outcome, possibly because of its effects on free radical production or cerebral metabolism.
- Admit the patient to hospital for assessment and rehabilitation – usually in a specialist stroke unit.
Patients have a risk of a further stroke within 5 years of between 30% and 43%, and also an increased risk of other cardiovascular events. Therefore, consideration needs to be given to lifestyle factors (smoking, diet and exercise), BP and cholesterol reduction. Stroke patients should have an annual immunisation against influenza.
Anticoagulants are usually prescribed for all patients with persistent or paroxysmal AF. There is a 68% reduction in the risk of stroke for patients with AF on warfarin compared with a 25–30% risk reduction with aspirin. Other patients with an ischaemic stroke require antiplatelet therapy such as low-dose aspirin (75 mg a day), clopidogrel (75 mg a day), or a combination of low-dose aspirin and modified-release dipyridamole (200 mg twice daily). Dipyridamole has both antiplatelet and vasodilating properties and is thought to inhibit the uptake of adenosine (a potent inhibitor of platelet activation and aggregation) into platelets and vascular cells. Dipyridamole may also inhibit the breakdown of cyclic guanosine monophosphate. For the mechanism of action of other antiplatelet drugs → p. 181.
Thrombolysis for CVA
Thombolysis with alteplase is proven to be beneficial if given within 4.5 h of an ischaemic stroke in selected patients. However, in Europe, alteplase is licensed to be given only in ischaemic stroke within 3 h of symptom onset. The aim is to limit the size of the cerebral infarct and thus improve the functional outcome. Alteplase (tPA) is given intravenously over 60 min in a dose of 900 mcg/kg (to a maximum of 90 mg). The initial 10% of the dose should be given by bolus injection and the remaining 90% by infusion. Thrombolysis is not yet proven to be of value for patients aged >80 years. A CT brain scan must be performed before thrombolysis to exclude intracerebral haemorrhage.
 Box 14.8 Specific Contraindications to the Use of Thrombolysis in Ischaemic Stroke
Box 14.8 Specific Contraindications to the Use of Thrombolysis in Ischaemic Stroke- Onset of symptoms more than 4.5 h previously or unclear time of onset
- Age <18 or >80 years
- Previous stroke within the past 3 months
- Previous stroke with concomitant diabetes
- Evidence of intracranial or subarachnoid haemorrhage
- Mild stroke or rapid improvement
- Very severe stroke
- Seizure at onset of stroke
- Systolic BP >185 mmHg or diastolic BP >110 mmHg
- Platelet count below 100 × 109/L
- Heparin within the last 48 h with raised PTT (prothrombin time)
Transient Ischaemic Attack
A TIA is defined as an abrupt loss of focal cerebral or monocular function with symptoms lasting <24 h which, after adequate investigation, is presumed to be caused by embolic or thrombotic vascular disease.
The risk of having a stroke after a hemispheric TIA is over 5% in the first 7 days, with the greatest risk occurring in the first 72 h. In addition, a TIA is a marker for general vascular disease (→ Box 14.9). Half of all TIAs are caused by the thromboembolic complications of arteriosclerosis in the arteries that supply the brain; emboli from the heart account for a further 20%.
 Box 14.9 Risk of Cardiovascular Diseases After a TIA
Box 14.9 Risk of Cardiovascular Diseases After a TIA- The average annual risk of stroke is about 7% (seven times the risk in the normal population)
- The greatest risk of a stroke is in the first year (half will occur in the 2 days after a TIA)
- The annual risk of MI is 2–3%
- The annual death rate from CVA, MI or other vascular death is about 9%
The diagnosis of TIA is based on the history. Symptoms – usually loss of function – are of sudden onset and are maximal from the start (→ Box 14.7). As TIAs are focal events, global symptoms such as light-headedness, dizziness and syncope are rarely consistent with the diagnosis. The following predisposing factors should be sought:
- AF or other source of cardiac embolism
- Hypertension
- Carotid disease (bruits)
- History of smoking
- Hypercholesterolaemia.
Investigations:
All patients should have a routine screen (FBC, ESR, U&Es, LFTs [liver function tests], glucose, random cholesterol, ECG and CXR). An urgent CT scan of the brain should be considered for patients aged <60 years and for those with atypical features, reasons for bleeding or an uncertain diagnosis. Carotid duplex scan is usually requested from the clinic by investigating physicians.
TX
Once the symptoms are clearly resolving, oral aspirin (300 mg) should be given to all patients in whom it is not contraindicated. Patients should be risk-assessed using the ABCD2 score (→ Box 14.10) and admitted to hospital if they have any of the following:
 Box 14.10 The ABCD2 Score
Box 14.10 The ABCD2 Score| Risk factor | Score |
| Age ≥60 years | 1 |
| Diabetes | 1 |
| Raised blood pressure (>140/90 mmHg) | 1 |
| Unilateral weakness | 2 |
| Speech impairment without weakness | 1 |
| Duration ≥60 min | 2 |
| Duration 1–59 min | 1 |
Other patients (i.e. ABCD2 score ≤4) may go home, provided that there are arrangements for assessment by a specialist service within 7 days of the TIA at the latest. The GP must be notified and aspirin (300 mg daily for 2 weeks and then 75 mg a day) prescribed for all patients (aspirin therapy reduces the incidence of CVA in patients who have had a TIA by around 3%/annum). Patients who are intolerant of aspirin (hypersensitivity or severe dyspepsia induced by low-dose aspirin) should be prescribed modified-release dipyridamole 200 mg twice daily. In the specialist clinic, the patient will be prescribed modified-release dipyridamole 200 mg twice daily in addition to aspirin or be changed to treatment with clopidogrel. If a patient is found to be in AF (and not anticoagulated), he or she should be prescribed aspirin 300 mg/day as above until seen in clinic.
Driving After a CVA or TIA
The UK Driver and Vehicle Licensing Agency (DVLA) rules on fitness to drive state that a patient with a diagnosis of CVA or TIA must not drive for at least 1 month. He or she may resume driving after this time if his or her clinical recovery is judged to be satisfactory and he or she has not had any fits. There is no need to notify the licensing authority (DVLA) unless there is a residual neurological deficit 1 month after the episode. A patient with multiple TIAs will require an attack-free period of 3 months before resuming driving and must notify the DVLA. This advice has obvious implications for patients discharged from an ED with a diagnosis of TIA.
Transient Global Amnesia
Transient global amnesia (TGA) describes a sudden and temporary loss of memory in an otherwise well patient who is fully conscious throughout the episode. Distant memories are preserved, as is immediate recall of current events. There is complete recovery within 24 h (and usually much sooner). TGA is of unknown aetiology and carries no increased risk of CVA or other illness although there is a small risk of recurrence. Intracranial venous congestion has been implicated in its causation but the main known risk factor is a history of migraine. Treatment centres around excluding other conditions and reassuring the patient.
Labyrinthitis (Vestibular Neuronitis)
Presumed viral infection of the inner ear or its nerve supply may cause an unpleasant condition closely related to motion sickness. The symptoms are the same:
- Profound nausea and vomiting
- Vertigo and ataxia
- Pallor, sweating and gross malaise.
The vertigo is positional and usually worsened by movements of the head and reduced by keeping still. Nystagmus may be present (fast component towards the affected side) but there is no hearing loss.
TX
Other conditions must be excluded before treatment is initiated. Bed rest is essential and affected patients cannot go home alone. The symptoms may persist for several days or even weeks, during which time the patient must not drive. (In its duration and dubious aetiology, vestibular neuronitis resembles another common cranial mononeuropathy, Bell’s palsy.)
If not contraindicated, the most effective drugs are:
Standard phenothiazines (e.g. prochlorperazine) and the serotonin 5HT3-receptor antagonists (e.g. metoclopramide) are relatively ineffective for vestibular conditions.
Other Vestibular or Cerebellar Signs
Sudden onset of ataxia must generally be referred to the physicians for investigation. Sometimes people with chronic alcohol problems and known cerebral degeneration present with an apparent disproportionate worsening of the cerebellar component of this disease. They should be admitted because the combination of alcohol, ataxia and the environment is often fatal.
Convulsions (Seizures)
Fits occur when there is one of the following:
- Deprivation of cerebral fuel supply (anoxic convulsion):
- hypoglycaemia or hypoxia
- transient dysrhythmia or cardiac arrest
- syncope
- hypoglycaemia or hypoxia
- Cerebral irritation:
- head injury
- cerebral tumour or infection
- poisoning
- fever (viraemia) in children
- primary epilepsy
- head injury
- Withdrawal of sedatives: alcohol or drug withdrawal.
A fit causes a dramatic increase in the cerebral metabolic demand for oxygen and also partial airway obstruction and decreased ventilation. Hypoxia must be prevented by urgent airway control and rapid treatment of the fit before elucidation of the underlying cause can be undertaken.
TX
- Protect or remove the patient from harm.
- Give high-concentration oxygen by facemask. Never attempt to force open the airway by inserting a wedge in the mouth – use a Guedel airway once the jaw has relaxed.
- Obtain venous access.
- Give IV diazepam emulsion (initially 5–20 mg for adults in incremental doses). If IV access cannot be obtained, then diazepam can be given rectally. Midazolam or lorazepam, at approximately a third of the dose recommended for diazepam, are useful alternatives.
When the fit has stopped:
- place the patient in the recovery position
- monitor vital signs
- check the blood sugar
- start investigating the circumstances that led to the fit and perform a full physical examination.
If the fit is not terminated within 30 min, the patient has status epilepticus (permanent damage is common, 10% mortality rate). Call for urgent help – an anaesthetist may be needed – and then consider the therapies below:
- Paraldehyde – 5–10 mL (up to 5 mL by deep IM injection into each buttock). This is useful if IV access is problematic.
- Phenytoin – immediate loading dose of 15 mg/kg by IV infusion at a rate of no more than 50 mg/min (or 1 mg/kg per min). Fosphenytoin sodium is a prodrug, which is converted to phenytoin immediately after administration. It is prescribed in milligram PE (phenytoin equivalents). Unlike phenytoin sodium, it dissolves freely in aqueous solutions and can be infused intravenously at rates up to three times faster than the parent drug (i.e. 100–150 mg PE/min). In the case of both drugs, the ECG must be monitored throughout the infusion and for at least 30 min afterwards.
- Phenobarbital – 15 mg/kg by IV infusion at a rate of not more than 100 mg/min.
As soon as it becomes apparent that more than one drug is required, consideration should be given to the need for general anaesthetic techniques. This usually entails either sedation with thiopental (up to 5 mg/kg) and then a thiopental infusion (1–3 mg/kg per h) accompanied by intensive care unit (ICU) monitoring or full anaesthesia, paralysis and ventilation.
Status epilepticus must be distinguished from serial seizures without full recovery. If the patient stops tonic–clonic seizures but does not regain full consciousness consider ‘absence’ or complex partial seizures. These are difficult to detect but important to stop because ongoing cerebral ischaemia is a strong possibility.
A fully recovered patient with known epilepsy may go home under the following circumstances:
- There are no unusual features in this episode.
- He or she remains well after 2 h observation.
- There is continuous social support both en route and at home.
- The GP is informed of the discharge.
- The usual drug supply is available.
Pseudo-Fits
The possibility of pseudo-fits must be considered. These are an attention-seeking device, often manifest in a public arena. They can be distinguished from genuine convulsions because:
- the patient is rarely injured
- there is no incontinence or tongue biting
- the plantar response remains flexor
- pulse oximetry remains normal, as opposed to the marked fall in saturation seen in a genuine fit.
Nevertheless, these patients cannot simply be dismissed. The pseudo-fit represents abnormal behaviour, often prompted by chronic and complex social problems. Discharge and follow-up should be discussed with the GP over the telephone.
Peripheral Neuropathies and Similar Conditions
There are many causes of failure of conduction of the peripheral nerves including drugs, diabetes, infections, vitamin deficiencies and alcoholism. Many cases are idiopathic. Guillain–Barré syndrome is the most common type of acute polyneuropathy in the UK → later.
Isolated peripheral neuropathies
may follow stretching or compression of nerves while intoxicated with alcohol or drugs. Radial nerve palsy (wrist drop) often occurs in these circumstances. Patients with foot drop sometimes present to an ED. Both of these conditions should be treated by splintage and referral for investigation.
Acute (transverse) myelitis
may look similar to a polyneuritis. It causes a specific sensory level with bilateral leg weakness and bladder and bowel dysfunction.
Multiple sclerosis (MS)
is not a peripheral neuropathy but may be mistaken for one because the multiple central nervous system (CNS) lesions responsible are scattered in both time and place. MS may present with a vast array of different neurological features including loss of vision, diplopia, spastic weakness of the legs, numbness and paraesthesia, sphincter disturbance, painful flexor spasms and cerebellar signs (vertigo, tremor and nystagmus).
Guillain–Barré Syndrome (Acute Polyneuritis)
Guillain–Barré syndrome is an ascending polyneuritis of unknown aetiology, which has an annual incidence of 1–2 cases per 100 000 throughout the world. It is the most common form of acute peripheral neuropathy in the UK and also the most common cause of acute neuromuscular paralysis. There are at least four clinicopathological subtypes. Most cases (90%) are described as ‘acute inflammatory demyelinating polyradiculoneuropathy’. Between 25 and 30% of patients require artificial ventilation. Mortality rates range from 3% to 6% overall but rise to around 20% in ventilated patients.
The features of Guillain–Barré syndrome develop over the course of a few days or sometimes several weeks. There may be a history of a preceding infection and then severe backache before the development of neurological symptoms and signs as below:
- Progressive, symmetrical, ascending weakness, which may include ocular, bulbar and respiratory muscles
- Diminished or absent tendon reflexes
- Variable sensory loss and pain (in up to 90% of patients)
- Autonomic disturbances such as tachycardia, cardiac dysrhythmias and labile blood pressure.
Motor involvement (which may be either proximal or distal) usually predominates over sensory loss (often distal). Of the cranial nerves, the facial nerve is the most commonly affected. Measurement of peak expiratory flow rate is useful to assess the degree of respiratory impairment. Very few investigations are helpful in the diagnosis of acute polyneuritis. The cerebrospinal fluid (CSF) usually contains an increased amount of protein.
Hyperacute cases of Guillain–Barré syndrome may resemble a stroke, cases with pain may masquerade as spinal cord (or cauda equina) lesions and pure motor cases may be confused with poliomyelitis, acute transverse myelitis, myasthenia or acute muscle disease.
TX
If the condition is clinically suspected, then the patient must be admitted to hospital. Intensive care is appropriate if there is bulbar or respiratory weakness or autonomic signs. Most cases of Guillain–Barré syndrome recover spontaneously over several weeks. Immunotherapy with intravenous immunoglobulin or plasma exchange has been shown to hasten recovery but corticosteroids do not help. Residual disability is found in 20% of those patients who survive the illness.
Cholinergic and Myasthenic Crises
In myasthenia gravis, too high a dose of anticholinesterase drugs may make the weakness worse (cholinergic crisis). This is easily mistaken for exacerbation of the primary disease (myasthenic crisis). Differentiation can be achieved by IV injection of edrophonium 2 mg (the ‘Tensilon’ test), which will induce a transient improvement in myasthenic crisis only.
TX
In both conditions, artificial ventilation may be required from the outset. For myasthenic crisis, IM neostigmine 0.5 mg should be given and repeated every 20 min as necessary. For cholinergic crisis, the treatment is pralidoxime 1–2 g by slow IV injection, again repeated every 20 min as required. The muscarinic effects of anticholinesterases must be controlled with IV atropine.
Tetanus
Cases of tetanus have increased in the UK during the past decade. Infection is caused by contamination of a wound with the spores of the bacillus Clostridium tetani. Days or even weeks after infection, local multiplication of the organism occurs with release of its neurotoxin. Even the most trivial of wounds can introduce tetanus into the body, but some wounds carry a particularly high risk of infection. Factors that help to identify tetanus-prone wounds → Box 21.6 on p. 396. The incubation period of tetanus is between 4 and 21 days (usually about 10 days) and the case fatality rate is almost 30%. Tetanus is a notifiable disease.
Active immunisation against tetanus was introduced into some areas of the UK as part of the primary immunisation of infants from the mid-1950s and was adopted nationally from 1961. As a result, tetanus virtually disappeared in children aged <15 years by the 1970s. Until recently, the majority of cases of tetanus in the UK were in people aged >65 years who had not been previously immunised. Two-thirds of the victims were women who, unlike most older men, had not been immunised during military service (immunisation against tetanus was provided by the British armed forces from 1938 onwards). However, in the last few years, men and women seem to be equally at risk from the disease. More recently still, tetanus has been seen in young adults who abuse IV drugs. (In the USA, 20% of tetanus cases are associated with IV drug abuse.) IM and subcutaneous injections are particularly likely to result in a suitable environment for spore germination because citric acid (which is used to dissolve heroin) may damage muscle tissue. Neonatal tetanus is still a major public health problem in many developing countries but there have been no cases in the UK for over 30 years. It is usually caused by infection of the umbilical stump as a result of poor hygiene.
It is important to understand the mechanism of action of tetanus toxin. In the ventral horn of the spinal cord, negative feedback loop neurons (Renshaw cells) limit the activity of the α-motoneurons. Tetanus toxin prevents the release of the inhibitory neurotransmitter glycine from these cells, which allows uncontrolled spasms of skeletal muscle. Strychnine (and brucine) compete with glycine for its receptors on the α-motoneurons and thus cause a very similar clinical picture. Acute dystonic reactions (‘oculogyric crises’) may also mimic tetanus → p. 300.
Figure 14.1 The role of the Renshaw cell in tetanus and in strychnine poisoning. Strychnine and brucine are alkaloids from plants of the genus Strychnos. Tetanospasmin is the neurotoxic component of the exotoxin produced by the tetanus bacillus. LMN, lower motor neuron; UMN, upper motor neuron.
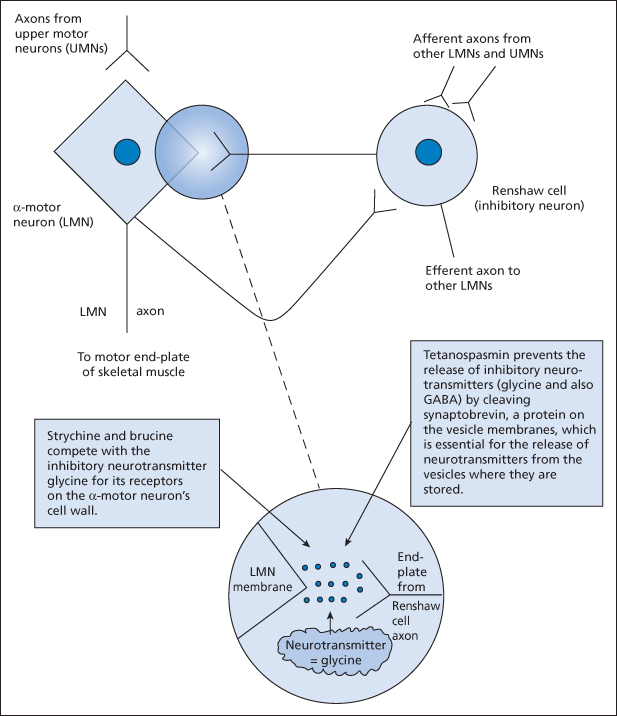
The clinical features of tetanus can progress slowly over as much as 2 weeks. In the usual order of appearance they are as follows:
- Stiff muscles near the wound (or injection site)
- Jaw stiffness and trismus (‘lock-jaw’)
- Abdominal rigidity
- Progressive painful muscle spasms (the fixed mocking grin caused by facial muscle tightening is known as the ‘risus sardonicus’ and extensor spasms of the back, neck and limbs are called opisthotonus)
- Dysphagia and respiratory difficulty
- Autonomic dysfunction.
TX
Supportive therapy is essential with special attention given to the airway, respiration and autonomic activities. IV benzodiazepines or even general anaesthesia may be required to control spasms. The tetanus bacillus is sensitive to both benzylpenicillin and metronidazole. Immunoglobulin against tetanus toxin (HTIg) 150 IU/kg is given intramuscularly in multiple sites (a range of doses from 30 IU/kg to 300 IU/kg has been suggested.) Tetanus immunoglobulin for IV use is also available on a named-patient basis. The dose is 5000–10 000 IU by IV infusion. Surgical debridement of wounds may help to reduce the toxin load. Suspicion of tetanus infection should provoke a discussion with a specialist in infectious diseases. Strychnine poisoning is treated with similar supportive and relaxant therapy.
Botulism
Botulinum toxin is one of the most poisonous substances known to humans. Several countries are known to have attempted weaponisation of the toxin for airborne dispersal leading to inhalation.
Food-Borne Botulism:
the features of botulism typically begin between 12 and 36 h after ingestion of contaminated food but may start after as few as 6 h. The earliest symptoms are usually gastrointestinal (nausea, vomiting and abdominal pain) and neurological (dysfunction of the cranial nerves).
Wound Botulism:
this variant of botulism has recently been seen in IV drug abusers, probably as a result of contaminated drugs and citric acid damage to muscle. Unlike the classic food-borne disease (which is caused by the ingestion of preformed toxin), wound botulism occurs when the spores of the anaerobic bacterium Clostridium botulinum contaminate a wound, germinate and produce toxin in vivo.
Botulinum toxin blocks the release of acetylcholine from the neuromuscular junction and characteristically causes a descending flaccid paralysis:
- Dysphonia, dysarthria and dysphagia (in >95% of cases)
- Blurred or double vision and ptosis (in >90% of cases)
- Dizziness and general muscle weakness (in >70% of cases)
- Immobility and respiratory distress.
Patients are afebrile with no sensory loss and no confusion. Autonomic signs include dry mouth, dilated pupils, constipation and urinary dysfunction; postural hypotension may also occur. Surprisingly, deep tendon reflexes are preserved. Diagnosis is confirmed in a reference laboratory using serum samples and wound swabs. Botulism is fatal in 10% of victims and those who recover are usually debilitated for many months.
TX
Ventilatory support may be required, as may surgical debridement of wounds. Clostridium botulinum is sensitive to benzylpenicillin and metronidazole. Botulinum antitoxin should always be given because it is effective in reducing both the severity and the duration of symptoms.
Conditions Caused by Diving
Decompression Sickness
Decompression sickness or caisson disease may occur up to 48 h after diving. It is caused by nitrogen (which has been dissolved in the blood under pressure) coming out of solution and forming bubbles in the bloodstream and tissues. The patient may present as a collapse without giving a history of recreational diving because many people do not appreciate the potential for delayed onset of symptoms. There is:
- malaise
- joint pains (the ‘bends’)
- itching and rashes
- lymphadenopathy and oedema.
In severe cases there are signs and symptoms of cardiopulmonary, vestibular and CNS involvement such as:
- chest pain
- dyspnoea
- coughing and haemoptysis
- hypertension or hypotension
- tinnitus or deafness
- nausea
- vertigo and nystagmus
- headache
- behavioural changes
- altered consciousness
- convulsions
- motor and sensory deficits, including hemiplegia, paraplegia and urinary retention.
TX
- Give oxygen
- Commence IV fluid resuscitation
- Control fits and agitation
- Discuss the patient with a specialist in diving and hyperbaric medicine. Recompression in a hyperbaric chamber may be required.
Nitrogen Narcosis and Oxygen Toxicity in Divers (‘Rapture of the Deep’)
Nitrogen Toxicity:
When breathed under pressure (at depths in excess of 100 feet or 30 m), nitrogen has an intoxicating effect. There is light-headedness, impairment of judgement and loss of coordination. Hallucinations, coma and death may occur at extreme depths. The narcosis reverses spontaneously on ascent to normal air pressures.
Oxygen Toxicity:
Breathing oxygen at high partial pressures (at depths over 200 feet or 60 m) causes CNS toxicity. Dizziness, nausea, disorientation, visual abnormalities and facial twitching occur; convulsions and coma can prove fatal. Treatment is supportive. Recompression requires careful oxygen monitoring because high inspired concentrations of oxygen may worsen the condition.
Barotrauma From Diving
The pressure changes associated with diving may also cause the following:
- Pneumothorax and surgical emphysema
- Air embolism (neurological signs may mimic decompression sickness)
- Rupture of the tympanic membrane (pain, bleeding, hearing loss)
- Disturbance of the inner ear (nausea, vomiting, vertigo, tinnitus and deafness may also look like decompression sickness)
- Pain around the sinuses and in the mouth (from air trapped in cavities beneath fillings or caries)
- GI pain, colic and flatulence.
If the clinical picture suggests a possibility of decompression sickness then the patient must be discussed with a hyperbaric specialist.
Altitude-Related Illness
Altitude, Hypoxia and Acclimatisation:
barometric pressure falls logarithmically with increasing altitude. The partial pressure of oxygen (21% of barometric pressure) falls at the same rate. At 19 000 feet (5800 m), these pressures are 50% of their values at sea level, falling to 28% at the summit of Mount Everest. The ambient temperature also drops by 6.5°C with every additional 3300 feet (1000 m) in altitude. The complex physiological changes of acclimatisation include increased minute ventilation, increased sympathetic tone, increased pulmonary vascular resistance and, after several weeks, an increased haematocrit.
Acute Mountain Sickness:
This affects around 75% of people who ascend rapidly to heights of more than 10 000 feet (3000 m) above sea level and even troubles 20% of people at just 7500 feet (2300 m). It is usually defined as a headache accompanied by any of the following symptoms: nausea, vomiting, malaise, fatigue, lethargy, anorexia and insomnia. Sleep may be disturbed by periodic breathing or even apnoea. Acute mountain sickness (AMS) usually resolves within 3 days of arriving at altitude but sometimes leads to life-threatening cerebral or pulmonary oedema. The causes of these altitude-related illnesses are poorly understood. There is some genetic predisposition associated with capillary leakage, fluid retention and stimulation of chemoreceptors in the presence of hypoxaemia. The risk is increased with rapid ascents and failure to acclimatise. All patients will have a low SaO2. It is said that AMS can be minimised by ‘climbing high and sleeping low’.
High-Altitude Cerebral Oedema:
the most severe manifestation of altitude sickness is acute cerebral oedema, which can be overwhelming if untreated. It is thought to have the same pathophysiology as AMS and may represent the extreme form of the condition. In addition to the usual symptoms of mountain sickness, there is a progressive deterioration in mental status with ataxia and, in some cases, focal neurological deficits. Eventually coma supervenes.
High-Altitude Pulmonary Oedema:
this is seen in 2–4% of climbers once 14 000 feet (4300 m) is reached and is the most common medical cause of death at altitude. Atmospheric hypoxia is thought to cause pulmonary hypertension, uneven perfusion and capillary leakage. The first symptoms are cough and tachypnoea, followed by increasing dyspnoea and cyanosis.
TX
Sufferers must be advised to stop and rest immediately before the condition worsens. Oxygen, analgesics and antiemetics should be given and descent arranged. A reduction in altitude of as little as 1500–3000 feet (450–900 m) may be all that is required. Acetazolamide 125–250 mg twice daily may be given to increase the rate of acclimatisation. High-altitude cerebral oedema is treated with dexamethasone 4 mg (by any route) every 6 h and hyperbaric therapy (in a portable unit such as a Gamow bag) if available. Patients with high-altitude pulmonary oedema should be given nifedipine 10 mg by mouth 4-hourly and hyperbaric therapy, in addition to standard treatments for the oedema.
Prophylaxis for AMS
Acetazolamide is a sulfonamide that inhibits carbonic anhydrase. It decreases renal bicarbonate reabsorption, causing a mild metabolic acidosis and also decreases the formation of CSF. Acetazolamide 125 mg twice daily is an effective prophylaxis for AMS that increases the rate of acclimatisation and reduces the severity of symptoms. Treatment should be started 2 days before ascent and continued for up to 5 days at altitude. The main side effects are diuresis and paraesthesiae. Chemoprophylaxis does not reduce the risk of altitude sickness; gradual ascent and physiological acclimatisation are still essential.
Other Risks in Climbers
These include hypothermia, frostbite, UV keratitis, exacerbation of pre-existing diseases and, of course, trauma.




