Immediate Assessment and Management
- Assess ABCs as outlined in Chapter 1.
- Reassure the patient.
- Allow the patient to sit or lie in whatever position is most comfortable.
- Obtain a 12-lead ECG immediately (within 5–10 min of arrival) and interpret it at once. If there are changes that, when combined with the clinical picture, indicate the need for reperfusion therapy (ST-segment elevation MI, left bundle-branch block or posterior MI) treat without delay (→ p. 181).
In any case, if there are any immediately obvious symptoms or signs of serious illness or if the patient looks unwell:
- Administer a high concentration of oxygen by mask if the SaO2 (arterial O2 saturation) is <94% on air.
- Connect the patient to monitoring devices – ECG, BP and SaO2.
- Obtain venous access and take blood for routine tests. Samples for cardiac markers should be taken at times dictated by local protocols (usually 12 h after the onset of pain for troponins) (→ p. 185).
- Request an urgent chest radiograph (CXR).
- Consider the need for early analgesia.
Further Assessment
Ask About
- Duration of pain
- Nature of pain including radiation and whether constant or intermittent
- Relationship of pain to breathing, coughing or moving
- Precipitating or relieving factors, including glyceryl trinitrate (GTN)
- Similarity of pain to previous events (e.g. MI or angina)
- Accompanying symptoms such as sweating, nausea, vomiting, flatus or general malaise
- Shortness of breath
- Collapse or blackout, however transitory
- Family and smoking history.
Look For
- Pallor, sweating or cyanosis
- Abnormal pulse or BP
- Raised jugular venous pressure (JVP) or dependent oedema
- Added heart sounds
- Changed respiratory rate and pattern
- Low SaO2 on air
- Reduced air entry and abnormal breath sounds
- Abdominal tenderness – especially epigastric and renal (which may mimic chest pain) and hepatic discomfort (congestive heart failure).
MI AND THE ACUTE CORONARY SYNDROMES
Coronary heart disease (CHD) is the most common cause of death and the single main cause of premature death in both men and women in the UK. It accounted for over a quarter of all deaths in England and Wales in 2010. By 2020, it is projected to be both the number one cause of death and the leading cause of life-years lost on a global scale. The three major risk factors for CHD are smoking, hypertension and hyperlipidaemia. The disease progresses faster in the presence of diabetes and obesity and doubles in incidence in those who are physically inactive. However, its victims are not all overweight, unfit individuals; MI strikes all shapes and sizes of people at almost all ages. Thirty per cent of people die from their first MI.
CHD begins several decades before the acute event with the formation of atherosclerotic plaques. Seventy-five per cent of fatal coronary thromboses occur when there is a sudden rupture of the thin fibrous cap covering an inflamed, lipid-rich plaque. Mechanisms such as plaque erosion account for the rest. The initial flow obstruction is due to platelet aggregation followed by vasospasm. This process is accelerated by the secretion of substances such as thromboxane A2, ADP and serotonin (5-hydroxytryptamine, 5HT) from entrapped platelets. Activation of the coagulation cascade then leads to the conversion of fibrinogen to fibrin, which is responsible for the subsequent stabilisation of the fragile thrombus. Within 30 min of complete coronary occlusion, MI develops, progressing from the subendocardium to the subepicardium.
The term ‘acute coronary syndromes’ (ACSs) describes a continuum of conditions that are associated with acute myocardial ischaemia as a result of interruption of the coronary blood supply. The syndromes range from unstable angina to full-thickness MI. ST-segment elevation MI (STEMI) exists as a distinct diagnostic entity because patients with specific ECG changes derive a unique benefit from immediate reperfusion therapy (thrombolysis or percutaneous coronary intervention [PCI]). Patients with other manifestations of ACSs are treated with low-molecular-weight heparins and antiplatelet therapy instead of fibrinolytic drugs (→ later).
Every year, nearly a quarter of a million people in England and Wales have an acute MI. Up to 50% of them die in the first month. Half of these deaths occur in the first 2 h. The GRACE (Global Registry of Acute Cardiac Events) risk score is recommended (by the National Institute for Health and Clinical Excellence or NICE) as an early method of predicting 6-month mortality and thus guiding further treatment. It divides ACS patients into low-risk (<3% mortality rate), intermediate-risk (3–8%) and high-risk (>8%) groups. An online GRACE risk score calculator is available.
Diagnosis of Acute Coronary Syndromes and Evolving MI
Symptoms
The pain is classically experienced in the central chest and described as crushing or heavy. Some patients say that it feels like a tight band around their chest; others describe it as like someone sitting on them. It is common for the patient to clench his or her fist as an aid to description. The pain usually lasts longer than 15–20 min. The radiation, if any, may be to the left arm, neck or jaw. There is some suggestion that, in right coronary artery blockage, the jaw is the site of radiation whereas, in left-sided occlusions, the pain is felt in the arm.
Accompanying symptoms such as sweating, pallor, nausea or general malaise are almost as helpful in making a diagnosis as the character of the pain. Coronary thrombosis may also present without pain (‘silent MI’), with atypical pain, with complications of MI (e.g. left ventricular failure or LVF) or with an acute confusional state in elderly people.
Symptoms of ‘Indigestion’:
Be aware of the middle-aged man with ‘indigestion’, especially if he has no past dyspeptic history of significance. A few days of vague retrosternal pain is not a history of dyspepsia. It is much more likely to be the warning pains that often predate an MI. Recurrent belching is a sign of autonomic disturbance and as such may accompany an infarction. Even symptomatic relief from antacids should not be relied on to point away from the heart.
Some patients present with very atypical pain, in both site and character. Examples of this would be pain localised to the abdomen or to the left shoulder or arm alone.
Signs
There are no invariable signs. Cardiogenic shock, acute pulmonary oedema or dysrhythmia makes the diagnosis much easier but physical examination of the patient is often normal. Pallor and sweating should never be ignored; they point to autonomic disturbance. The heart rate may be fast, slow or average. Likewise, the BP may be raised or low but is often normal. A slow heart rate often occurs in an inferior MI because the right coronary artery that supplies this area also supplies the conducting system. (In addition, it delivers blood to the posterior myocardium and the right ventricle, although in some patients the circumflex branch of the left coronary artery is responsible for much of this territory. The anterior and lateral myocardium is invariably supplied by the left anterior descending artery.)
Investigations in MI / ACS
- Immediate ECG (within a few minutes of arrival) is essential. Repeat ECG after 30–60 min may also be helpful.
- CXR is usually normal.
- Routine blood tests should be taken, especially a blood glucose to identify the need for a glucose and insulin infusion in ACS and MI.
- A reduction in pain after the administration of GTN may be a helpful test but unfortunately this effect also occurs in oesophageal spasm.
More advanced methods of diagnosis of myocardial ischaemia exist and some are undergoing clinical trials:
- Multi-lead ‘vest’ ECGs, which use over 80 chest electrodes
- High-resolution CT
- Two-dimensional echocardiography – regional motion abnormalities of the myocardium develop seconds after coronary occlusion
- Technetium scanning – a normal resting technetium-99 myocardial perfusion scintigram effectively excludes major MI. However, an abnormal scan is not diagnostic.
ECG Diagnosis of Coronary Thrombosis and Evolving Infarction Requiring Immediate Reperfusion Therapy
Complete coronary occlusion causes an evolving MI that can be averted by timely reperfusion therapy. However, only when chest pain is accompanied by three specific ECG patterns has immediate reperfusion been shown to be of benefit. These three patterns are:
(The term STEMI is useful in the acute situation to determine treatment and is analogous to older descriptions such as Q-wave MI and transmural infarction.)
1 ECG Changes in STEMI
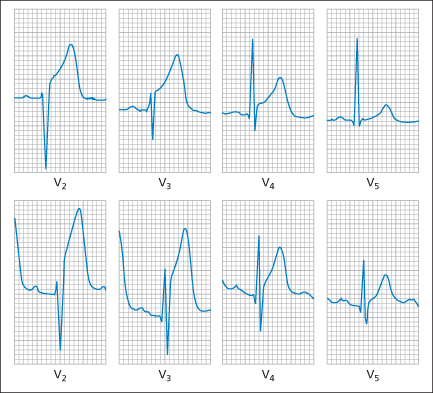
ST-segment elevation signifies complete occlusion of a major coronary artery and accounts for 30–40% of classic MIs. The occlusion/infarction sequence classically begins with subtle alterations in the T wave. These hyperacute T waves are tall, peaked and broadened with a loss of clear demarcation from the ST segment (→ Figure 12.1). Elevation of the ST segment (‘convex-upwards’) soon follows in leads that face the area of injury; reciprocal ST depression may also occur (→ Figure 12.2). Abnormal Q waves, which signify myocardial necrosis, may be present in the first ECG but in most cases do not appear for hours or even days after presentation. These pathological Q waves are wide (0.04 s – one small square – or more) and deep (4 mm or a quarter of the size of the subsequent R wave). They result from potentials that are transmitted through an electrical window of inexcitable myocardium and so suggest the presence of complete (transmural) rather than partial-thickness (subendocardial) damage (→ later). Severe intracellular disturbance may cause a similar picture. Finally, as the ST segment returns to the baseline, symmetrically inverted T waves appear. The timing and magnitude of all of these ECG changes vary greatly from patient to patient.
The site of a typical transmural infarction may be localised from the position of the ECG changes:
- Anterior: V1, V2, V3, V4
- Lateral: V5, V6
- High lateral: I, aVL
- Inferior: II, III, aVF (additional ST elevation in V4R [a right-sided V4] suggests infarction of the right ventricle → Box 12.1 and p. 189).
 Box 12.1 ECG Signs of True Posterior and Right Ventricular MI
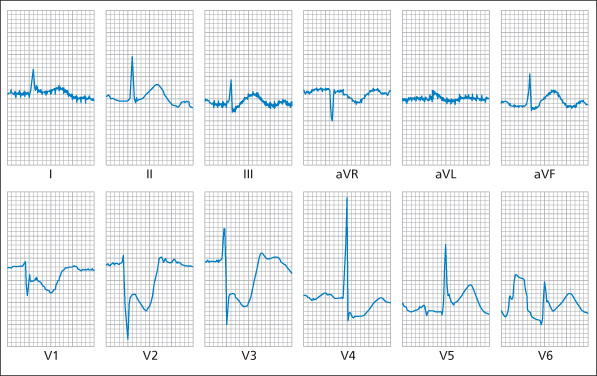
Box 12.1 ECG Signs of True Posterior and Right Ventricular MI- Tall R wave in the anterior leads (V1–V3)
- ST depression in the anterior leads (V1–V3), i.e. there is a mirror image of the usual Q wave and ST elevation pattern (→ Figure 12.3)
- ST elevation in posterior chest leads (→ Figure 12.4)
- ST elevation in V4R
- ST elevation in V1–V3 (and eventually Q waves in these leads)
- ECG changes of inferior MI
ECG changes must meet defined criteria for the diagnosis of STEMI and the initiation of reperfusion therapy:
2 ECG Changes in True Posterior MI
Similar to conventional STEMI, true posterior infarction results from complete occlusion of a major coronary artery and, if untreated, leads to irreversible myocardial necrosis. The term ‘true’ refers to the isolation of the infarct to the posterior myocardium as opposed to being part of a posterolateral or inferoposterior picture.
Figure 12.3 shows a 12-lead ECG of a posterior infarction.
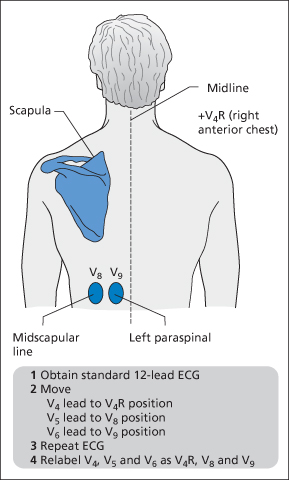
However, although an isolated posterior infarct may be hard to diagnose on a conventional 12-lead ECG, it can be more reliably seen on a 15-lead or a 17-lead ECG. A 15-lead ECG has been described that utilises V4R and two posterior leads (→ Figure 12.4). This technique increases the detection of ST elevation in MI by about 10%. The extra three leads should be obtained when the standard 12-lead ECG shows either ST depression in the anterior chest leads or equivocal right-sided or inferior changes. Low-voltage ST elevation of just 0.5 mm in the posterior chest leads is sufficient to diagnose a posterior STEMI and initiate reperfusion therapy.
3 ECG changes in LBBB with MI
Patients with LBBB have a much worse prognosis with acute MI than patients with normal ventricular conduction, whether the LBBB is pre-existing or new. However, these patients (who account for around 0.5% of all patients with infarcts) have been shown to benefit greatly from reperfusion therapy. Consequently, in the presence of LBBB, it is essential to diagnose an MI promptly. This is difficult because the normal sequence of ventricular activation is altered and there is ST elevation because of the LBBB itself. Three ECG criteria (the Sgarbossa criteria) have been shown to have independent value in the diagnosis of MI in patients with LBBB (→ Box 12.2). Unfortunately, these criteria have to be scored and added to be useful and do not apply to all MIs with LBBB. Therefore, all patients with chest pain and proven or suspected new LBBB should be treated with PCI or fibrinolytic therapy without delay.
 Box 12.2 ECG Diagnosis of MI in the Presence of LBBB
Box 12.2 ECG Diagnosis of MI in the Presence of LBBBTreatment of ST Elevation MI (STEMI)
Having made the diagnosis of an evolving MI requiring immediate reperfusion therapy, the emergency treatment detailed below must not delay preparations for thrombolysis or percutaneous coronary intervention (PCI). Proceed immediately to reperfusion therapy without completing the following list:
- Oxygen by mask and IV access.
- Continuous monitoring of vital signs and ECG.
- Analgesia – IV morphine 5–10 mg or diamorphine 2.5–5 mg with an antiemetic (e.g. IV metoclopramide 10 mg).
- Antiplatelet therapy – aspirin 300 mg by mouth and oral clopidogrel. Aspirin is as effective as (and additive to) fibrinolysis in reducing death rates in STEMI. After an MI, it is continued for life at a dose of 75 mg daily. There are few contraindications to aspirin – known hypersensitivity, bleeding peptic ulcer and severe liver disease. Clopidogrel 300 mg by mouth should also be given to all patients aged <75 years with STEMI, including many of those in whom aspirin is contraindicated. However, in patients aged >75 years with STEMI, the dose of clopidogrel is omitted or reduced to 75 mg. Anti-platelet agents seem to have a synergistic effect with fibrinolytic drugs. For those patients who are having primary PCI, most centres routinely prescribe clopidogrel 600 mg or, alternatively, the newer thienopyridine antiplatelet agent prasugrel 60 mg by mouth. Prasugrel is not recommended for patients who are aged >75 years, weigh <60 kg or have a history of transient ischaemic attack (TIA) or stroke.
- Nitrates – nitrates are used for persistent pain. Buccal GTN is a convenient and effective alternative to IV nitrates. The initial dose is 2 mg but this may be increased to 3–5 mg if necessary in the carefully monitored patient. A sublingual aerosol spray of GTN can be useful for a quick effect (two puffs = 800 mcg). For GTN infusions → p. 209.
- Glucose and insulin infusion – patients with diabetes and MI have double the mortality of those who do not have diabetes. In addition, there is experimental and limited clinical evidence that routine administration of glucose and insulin may improve metabolism in ischaemic myocardium. This treatment is inexpensive and should be given to all patients with either known diabetes or a blood sugar >11 mmol/L on admission. For variable rate IV insulin regimen → Box 14.14 on p. 242.
- β Blockers – if not contraindicated, early administration of a β blocker is recommended for all patients with MI. IV atenolol 5 mg is given over 5 min and the dose repeated after 10–15 min; 25 mg by mouth is an alternative. IV metoprolol is also licensed for this indication.
Reperfusion Therapy for STEMI
There are two possible first-line reperfusion treatments:
Local factors and protocols will control which of the above two treatments is most suitable for a particular patient. The availability of interventional cardiac catheterisation facilities is usually a major limiting factor in the UK. PCI is always the treatment of choice in cases where thrombolysis is contraindicated or has obviously failed. If the patient’s chest pain started more than 3 h before arrival, (a delayed ‘pain-to-needle’ time), then primary PCI is much more effective than thrombolysis. PCI is contraindicated in severe thrombocytopenia. In the prehospital situation, fibrinolytic drugs offer the only possible means of reperfusion.
PCI is better at opening closed arteries than thrombolysis. It achieves TIMI (‘thrombolysis in myocardial infarction’) grade 2 or 3 flow in over 95% of cases whereas fibrinolytic drugs have a maximum success rate of around 55% at completely opening up the artery responsible for the STEMI (TIMI grade 3 flow). Primary PCI is also associated with lower rates of stroke and non-fatal reinfarction than thrombolysis and goes some way to treating the underlying condition. It is much superior for patients with cardiogenic shock. When compared with tenecteplase or alteplase given within 2 h of symptom onset, very early PCI saves the lives of about 10 patients per 1000 treated. However, the added time to accomplish angioplasty often negates this advantage. Fibrinolytic drugs are most effective within 2–3 h of symptom onset. Within 6 h of the onset of chest pain, their administration prevents 30 deaths per 1000 patients treated (with aspirin increased to 50/1000 patients). Between 7 and 12 h, this reduces to 20 lives saved per 1000 patients treated. After 12 h, the risk–benefit ratio for fibrinolytic drugs is less certain. Thrombolysis increase mortality if given more than 24 h after symptom onset or to patients with ST depression or T-wave inversion without ST elevation.
Fibrinolytic drugs cause around 4 extra strokes per 1000 patients treated. These occur in the first 24 h, two patients dying and two surviving, one of whom will be disabled. The risk of stroke is predicted by advanced age, low body weight, female gender, previous cerebrovascular accident (CVA) and hypertension, especially hypertension on admission. Major non-cerebral bleeds occur in 7/1000 patients treated and are most commonly procedure related. Again advanced age, low body weight and female gender are associated factors.
The resolution of chest pain and reduction in ST elevation suggest reperfusion, as does the emergence of arrhythmias. Failed reperfusion (i.e. <50% reduction in ST elevation on ECG within 90 min of starting the treatment) is an indication for rescue PCI. There is little evidence for re-thrombolysis except after the less effective drug streptokinase. Coronary artery bypass graft (CABG) surgery is becoming more common as an emergency procedure in the UK.
Thrombolytic Therapy for STEMI
Fibrinolysis is an endogenous process whereby the insoluble fibrin that binds a blood clot together is broken down into soluble fragments (fibrin degradation products or FDPs). It is initiated by plasminogen activators such as tissue plasminogen activator (tPA), which are secreted into the circulation by the vessel wall. These substances convert plasminogen, a protein normally present in the plasma, into plasmin, which digests fibrin. Free circulating plasmin is inactivated by α2-antiplasmin. Fibrinolytic drugs exert their effect by activating plasminogen to form plasmin, which degrades fibrin, so breaking up thrombi.
The criteria for the administration of thrombolytic therapy are as follows:
- ST elevation of at least 2 mm in two adjacent precordial leads (V2–V6)
- ST elevation of at least 1 mm in two limb leads (I, II, III, aVL or aVF)
- LBBB (presumed or proven to be new)
- evidence of true posterior infarction (→ Box 12.1 and Figure 12.3). Low-voltage ST elevation of just 0.5 mm in the posterior chest leads is sufficient to diagnose STEMI
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree