Clinical Classifications of Pain
Acute pain: Pain caused by noxious stimulation from injury, a disease process, or the abnormal function of muscle or viscera. Nociceptive pain serves to detect, localize, and limit tissue damage.
Somatic pain: Further classified as superficial or deep
• Superficial somatic pain is caused by nociceptive input arising from skin, subcutaneous tissues, and mucous membranes. Characteristically well localized and described as a sharp, pricking, throbbing, or burning sensation.
• Deep somatic pain arises from muscles, tendons, joints, or bones. Pain usually has a dull, aching quality and is less well localized.
Visceral pain: Visceral acute pain is caused by a disease process or abnormal function involving an internal organ or its covering (e.g., parietal pleura, pericardium, or peritoneum).
• Four subtypes are described:
1. Localized visceral pain: Dull, diffuse, and usually midline. It is frequently associated with abnormal sympathetic or parasympathetic activity, causing nausea, vomiting, sweating, and changes in blood pressure and heart rate.
2. Localized parietal pain: Parietal pain is typically sharp and often described as a stabbing sensation that is either localized to the area around the organ or referred to a distant site.
3. Referred visceral pain and (4) referred parietal pain: The phenomenon of visceral or parietal pain referred to cutaneous areas results from patterns of embryologic development and migration of tissues and the convergence of visceral and somatic afferent input into the central nervous system (CNS).
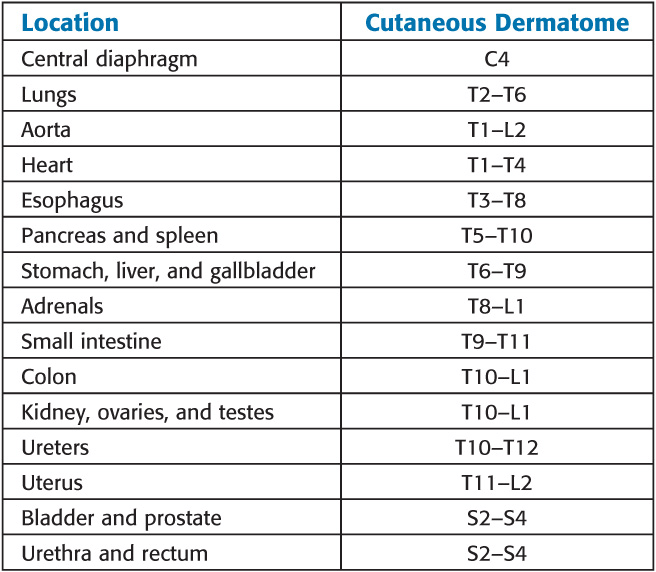
Patterns of Referred Pain
Chronic pain: Defined as pain that persists beyond the usual course of an acute disease or after a reasonable time for healing to occur (1–6 months). May be nociceptive, neuropathic, or mixed. Neuropathic pain is classically paroxysmal and lancinating, has a burning quality, and is associated with hyperpathia. When the sympathetic system plays a major role, it is termed sympathetically maintained pain.
Physiology of Nociception
Nociceptors
• Characterized by a high threshold for activation and encode the intensity of stimulation by increasing their discharge rates in a graded fashion.
• Noxious sensations can often be broken down into two components:
° First pain: Fast, sharp, and well-localized sensation, which is conducted with a short latency (0.1 sec) Aδ fibers (tested by pinprick)
° Second pain: More dull, slower onset, and often poorly-localized, which is conducted by C fibers
• Most nociceptors are free nerve endings that sense heat and mechanical and chemical tissue damage.
Cutaneous Nociceptors
• Nociceptors are present in both somatic and visceral tissues.
• Primary afferent neurons reach tissues by traveling along spinal somatic, sympathetic, or parasympathetic nerves.
• The cornea and tooth pulp are unique in that they are almost exclusively innervated by nociceptive Aδ and C fibers.
Deep Somatic Nociceptors
• Deep somatic nociceptors are less sensitive to noxious stimuli than cutaneous nociceptors but are easily sensitized by inflammation.
• The pain arising is described as dull and poorly localized.
Visceral Nociceptors
Visceral organs are generally insensitive tissues that mostly contain silent nociceptors.
Afferent fibers are free nerve endings whose cell bodies lie in the dorsal horn.
Afferent activity from these neurons enters the spinal cord between T1 and L2. Nociceptive C fibers from the esophagus, larynx, and trachea travel with the vagus nerve to enter the nucleus solitarius in the brainstem. Afferent pain fibers from the bladder, prostate, rectum, cervix and urethra, and genitalia are transmitted into the spinal cord via parasympathetic nerves at the level of the S2 to S4 nerve roots.
Chemical Mediators of Pain
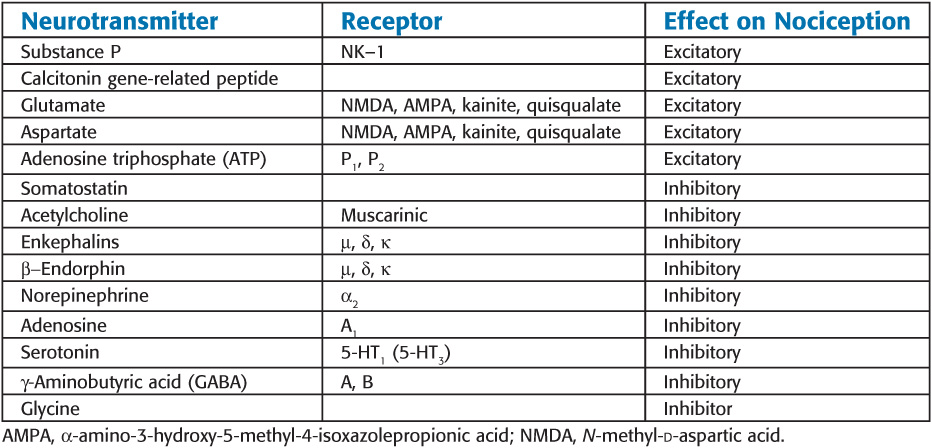
Major Neurotransmitters Mediating or Modulating Pain
Modulation of Pain
Occurs peripherally at the nociceptor, in the spinal cord, and in supraspinal structures. This modulation can either inhibit (suppress) or facilitate (intensify) pain.
Peripheral Modulation of Pain
1. Nociceptors and their neurons display sensitization after repeated stimulation.
2. Sensitization may be manifested as an enhanced response to noxious stimulation or a newly acquired responsiveness to a wider range of stimuli, including nonnoxious stimuli.
3. Primary Hyperalgesia
a. Sensitization of nociceptors results in a decrease in threshold, an increase in the frequency response to the same stimulus intensity, a decrease in response latency, and spontaneous firing even after cessation of the stimulus
b. Commonly occurs with injury and following application of heat
c. Mediated by the release of noxious substances from damaged tissues.
4. Secondary Hyperalgesia (neurogenic inflammation)
a. Manifested by the “triple response” of a red flush around the site of injury (flare), local tissue edema, and sensitization to noxious stimuli
b. Primarily caused by antidromic release of sP (and probably calcitonin gene-related peptide) from collateral axons of the primary afferent neuron
• Facilitation: Three mechanisms are responsible for central sensitization in the spinal cord:
1. Wind-up and sensitization of second-order neurons
2. Receptor field expansion
3. Hyperexcitability of flexion reflexes
° Glutamate and aspartate play an important role in wind-up, via activation NMDA and non-NMDA receptor mechanisms. These amino acids are believed to be largely responsible for the induction and maintenance of central sensitization.
• Inhibition: Transmission of nociceptive input in the spinal cord can be inhibited by segmental activity in the cord itself, as well as by descending neural activity from supraspinal centers.
° Segmental inhibition: Gate theory supported by
1. Activation of large afferent fibers subserving sensation inhibits wide dynamic range (WDR) neuron and spinothalamic tract activity.
2. Activation of noxious stimuli in noncontiguous parts of the body inhibits WDR neurons at other levels
Supraspinal Inhibition
• Several supraspinal structures send fibers down the spinal cord to inhibit pain in the dorsal horn.
• Origin for these descending pathways include the periaqueductal gray, reticular formation, and nucleus raphe magnus (NRM)
Pathophysiology of Chronic Pain
Chronic pain is caused by a combination of peripheral, central, and psychological mechanisms.
The sympathetic nervous system plays a major role. Disorders that respond to sympathetic blocks include complex regional pain syndrome, deafferentation syndromes caused by nerve avulsion or amputations, and postherpetic neuralgia
Systemic Responses to Pain
• Acute Pain
° Moderate to severe acute pain, regardless of site, can affect nearly every organ function and may adversely affect perioperative morbidity and mortality
° Effects
• Cardiovascular: Hypertension, tachycardia, enhanced myocardial irritability, increased systemic vascular resistance, and increase in myocardial oxygen demand; may precipitate myocardial ischemia
• Respiratory: Increase total body O2 consumption and CO2 production
• Gastrointestinal and urinary: Enhanced sympathetic tone increases sphincter tone and decreases intestinal and urinary motility, resulting in ileus and urinary retention
• Endocrine: Increases catabolic hormones (catecholamines, cortisol, and glucagon) and decreases anabolic hormones
• Chronic Pain
° Neuroendocrine stress response observed only in patients with severe recurring pain.
° Sleep and affective disturbances, particularly depression, are often prominent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





