61 Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) is a major cause of death and disability worldwide and is one of the most common reasons for intensive care unit (ICU) admission. Several monographs review this complex disorder in some detail.1,2 The intensivist’s view of COPD is predominantly physiologic, focusing on the impact of disrupted function on the individual’s normal homeostatic mechanisms. Although many important insights that have shaped our understanding of COPD have come from ICU studies, other aspects of this disorder must be considered if a rational approach to COPD management is to be developed.
Access to ICU care for sick COPD patients remains relatively inequitable among different healthcare systems. In North America and parts of Western Europe, most patients are offered ICU care, but in other relatively developed healthcare systems, such as in the United Kingdom, this is not the case. Even physicians in the same healthcare system differ significantly in their selection of patients for ICU referral.3 These choices may be influenced by local resource availability, but they are also conditioned by the generally pessimistic view of the outcome achievable with this treatment intervention. However, poor response to treatment in the ICU is not universal, and extended periods of mechanical ventilation are not invariably required to successfully manage patients with COPD.4 Nevertheless, intensivists often take a particularly bleak view of the prognosis of COPD patients compared with others entering their units. In one prospective study, intensivists estimated the survival of the sickest COPD patients to be 10% at 180 days post admission, when in fact it was 40%.5 In a survivor population after mechanical ventilation, 96% were happy to have received ventilator support, despite their continuing physical problems.6 Clearly, decisions about ventilator support should not be made in the emergency department without sufficient medical information or a proper discussion with the family. Supportive therapy should be offered until it is clear what the patient’s wishes are and what the likely outcome of treatment will be.
 Definition and Natural History
Definition and Natural History
“Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease with some extrapulmonary effects that may contribute to severity in individual patients. Its pulmonary component is characterized by airflow limitation that is not fully reversible. The airflow limitation is usually both progressive and associated with an abnormal inflammatory response of the lungs to noxious particles or gasses.”7
The emphasis here is on incompletely reversible airflow obstruction that is persistent and progressive. Symptoms and disability usually parallel these processes, although some individuals can apparently cope with a severe degree of airflow limitation without seeking medical help. Such patients finally present to the emergency room when they develop a severe exacerbation of COPD. In this situation, it is wisest to offer ventilatory support until the patient has at least had a chance to improve with conventional medical therapy. More common is a patient whose progressive illness is accompanied by repeated exacerbations, events that identify an accelerated decline in both lung function and health status.8,9 Such patients have often been hospitalized previously, and their response to treatment is usually clearly established.
The usual inhaled particles or gases that produce COPD are a complex mixture of hydrocarbons and particulates derived from tobacco smoke. These are the principal causes of COPD in the United States and western Europe,10 although other factors such as poor lung function during childhood, bronchial hyperresponsiveness, and low birth weight may also be important. The associated inflammatory changes, which persist when smoking stops,11,12 are thought to explain the airway and parenchymal destruction and fibrosis within the lung, although this has not been conclusively established as the only mechanism.
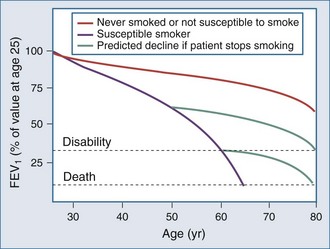
The natural history of COPD explains why the number of patients presenting for ICU care has not diminished in the last 3 decades as might be expected, given the overall reduction in tobacco consumption in Western countries. This is illustrated by the classic study of Fletcher and Peto, which has now been confirmed by longitudinal data from the Framingham study13,14 (Figure 61-1). Although the rate of decline of lung function is reduced in individuals who stop smoking, the lung function already lost is never regained, and even if the rate of decline of lung function returns to normal, these patients are still more likely to experience disability as they age. Thus, in an aging population that contains many former smokers, a significant number will still develop complications of COPD that require ICU care. The situation is complicated by the steadily rising number of women who smoke.15 Women are at least as susceptible as male smokers and more likely to be symptomatic. Thus an early fall in the number of COPD cases is being offset by the changing demographics of the current and ex-smoking population.
The important role of comorbidities in COPD has now been recognized.16 Most patients with significant symptoms due to COPD have at least one if not many comorbid diseases, especially cardiovascular problems.17 Whether the association is causal or an epiphenomenon is of little relevance in the ICU, where a high index of suspicion for undiagnosed comorbid disorders is a useful aid to effective management.
 Pathology
Pathology
The pathologic features of COPD depend on the stage of the illness and the part of the lung examined.18 Central airways show mucous gland hypertrophy and goblet cell metaplasia, whereas more peripheral airways show variable combinations of smooth muscle hypertrophy, peribronchial fibrosis, luminal occlusion by mucus, and enlarged lymphoid follicles. Alveoli are often but not invariably enlarged by the loss of alveolar walls, with an attendant loss of support for the small non-cartilaginous airways in this region of the lung. There is evidence of persistent inflammation, with neutrophils in the airway lumen and macrophages in the airway wall. CD8+ T lymphocytes are more prominent in this response than in bronchial inflammation of an asthmatic type, although intermediate states appear to exist.19 Inflammatory cells are also present adjacent to breaks in the alveolar wall.20 Overall, as the clinical and spirometric severity of the disease increases, so do the numbers of each cell population involved in the inflammatory process.21 In addition, extraluminal lymphoid follicles develop containing CD4+ lymphocytes, possibly reflecting a response to repeated infective exacerbations.21 Data obtained during exacerbations, though limited, support an increased role for neutrophils and, surprisingly, eosinophils.22
 Physiology
Physiology
The pathologic changes just described combine to produce the characteristic diagnostic finding of reduced forced expiratory flow (FEV1) at a given lung volume, which is usually assessed on a time base as an FEV1/forced vital capacity (FVC) ratio of less than 0.7. Technically, this should be 70% of the age-adjusted normal value for this ratio, because lung elastic recoil declines with age, even in healthy individuals. Use of the uncorrected ratio tends to overdiagnose COPD among the very elderly.23 In practice, however, this does not cause problems for COPD patients admitted for ICU care, because they are invariably more severely affected.
COPD affects all aspects of lung function, but its primary impact is a change in lung mechanics. This is traditionally analyzed in terms of the static (no flow) and dynamic (flow) properties of the respiratory system.24 Because chest wall mechanics are believed to be normal in COPD (although they are seldom measured directly), changes in the pressure-volume characteristics of the respiratory system are determined by alterations in lung compliance, often attributed to the loss of elastic recoil due to emphysema. How large a role this plays in changes in tissue compliance is not known. The resulting steeper slope, early-onset inspiratory plateau, and increase in end-expiratory lung volume are typical of the pressure-volume relationships in patients with COPD. Changes in end-expiratory lung volume and increases in residual volume change chest wall geometry favor a lower, flatter diaphragm and a more horizontal rib cage; these changes, in turn, impair the inspiratory muscles’ ability to develop pressure, and increase the overall work of breathing.25 Expiratory muscle activation is common in more severe COPD26,27 even at rest, and provides a useful clinical marker of respiratory distress. Flattening of the diaphragm redirects the axis of shortening of the skeletal muscle and often produces paradoxical in-drawing of the lower thoracic rib cage (so-called Hoover’s sign), which becomes more evident as pulmonary hyperinflation and respiratory drive to breathe rise. Patients with Hoover’s sign are more breathless and have more hyperinflation of their chest wall during exercise.28
The dynamics of the respiratory system are influenced by static properties but also differ significantly between inspiration and expiration. Maximum inspiratory flow is affected by inspiratory resistance as well as by the inspiratory muscles’ ability to develop pressure (and thus indirectly by chest wall geometry). Maximum expiratory flow is influenced by expiratory pressure generation and, more importantly, by the onset of volume-related airflow limitation, best described by the maximum expiratory flow-volume loop. As lung volume falls during expiration, airways close or become flow limited; hence, the flow at a specific lung volume is reduced. Although an assessment of flow (FEV1) relative to total volume change during expiration (FVC) is useful in defining COPD, an assessment of tidal flow limitation is more helpful in determining the degree of dyspnea experienced by the patient.29 More attention is now being paid to the determination of expiratory flow limitation under tidal conditions. In the past, detection was difficult, involving invasive measurements or reliance on body plethysmography, which tended to overestimate the incidence of tidal expiratory flow limitation. The development of the negative expiratory pressure test and, more recently, within-breath variation in respiratory system reactance has changed this.30 The within-breath method assesses more breaths, is less prone to observer error, and is likely to be automated in future for ICU application.31
In general, the lower the FEV1, the greater the likelihood that expiratory flow limitation is present. However, some COPD patients are not flow-limited on every breath and regulate their end-expiratory lung volume to try to minimize this. When respiratory drive rises (e.g., during exercise), during disease exacerbations, or when minute ventilation has to increase to maintain gas exchange during ventilator weaning, this resting variation in expiratory lung volume is likely to decrease. If expiratory flow and hence tidal volume are to increase, end-expiratory lung volume must rise; this further increases the work of breathing and the sensation of respiratory distress. This process, described as dynamic hyperinflation, has been clearly demonstrated during exercise and can be lessened by bronchodilator treatment which aids lung emptying.32
In the ICU, patients have a high respiratory drive during weaning and adopt a rapid, shallow breathing pattern. Total respiratory muscle work increases, in part because of the increased operating lung volumes, but also because of the presence of intrinsic positive end-expiratory pressure (PEEPi). This represents the pressure that must be developed to overcome residual expiratory driving pressure before inspiratory flow can begin.33 Calculating the size of this variable is fraught with technical difficulties beyond the problems of accurate placement of the balloon catheter system in intubated patients. Several methods have been proposed that correct for the effects of coexisting abdominal muscle activation, with recent work favoring a correction based on the total decay of gastric pressure.34 However, the need to compute this variable in clinical practice has been questioned.35
Gas Exchange
Arterial hypoxemia is common in COPD but becomes clinically significant only when the partial pressure of oxygen in arterial blood (PaO2) falls below 60 mm Hg, a problem largely confined to patients with an FEV1 below 35% of their predicted value. It arises predominantly due to ventilation-perfusion mismatching, often worsens during exercise, and is readily corrected by a small increase in the inspired oxygen concentration, unless the situation is made worse by secretion retention or severe pneumonia.36 Arterial hypercapnia is seen in some but not all hypoxemic patients who are clinically stable, but it is more frequent, at least temporarily, in hospitalized individuals.37 A combination of ventilation-perfusion mismatching due to an increase in physiologic dead space and a degree of effective alveolar hypoventilation explains this phenomenon. Acute rises in the partial pressure of arterial carbon dioxide (PaCO2) precipitate respiratory acidosis, a more reliable guide to prognosis and the need for ventilation than the PaCO2 itself.38,39
Control of Breathing
Despite years of study, there is no conclusive evidence that ventilatory control is abnormal in COPD patients. However, the response to sustained mechanical loading appears to be variable in healthy subjects40 and may explain why some individuals adopt the breathing patterns they do. Traditional techniques of studying respiratory control, which involve stimulation with exogenous CO2 or nitrogen, suggested that respiratory drive was reduced. However, studies using mouth occlusion pressure techniques or recording the electrical activation of inspiratory muscles suggest that respiratory drive is generally high, even in those COPD patients who tolerate relatively high levels of CO2.41–43 Studies of breathing pattern have been more instructive. In general, the lower the tidal volume, the higher the PaCO2.44 This is because the ratio of dead space (its fixed, predominantly anatomically determined volume) to tidal volume increases as the latter is reduced. Small tidal volumes are accompanied by an increased respiratory frequency to maintain the somewhat higher-than-normal level of minute ventilation. The resulting shortening of inspiratory time is also associated with hypercapnia.44 The system appears to be regulated to minimize peak inspiratory pressure generation, even at the cost of impaired gas exchange. There are theoretical reasons for believing that this is both energy efficient and likely to minimize the occurrence of inspiratory muscle fatigue.45 This also explains the usefulness of rapid, shallow breathing as an index of weaning failure when neuromechanical coupling in the respiratory system is under considerable stress.46
Pulmonary Circulation
In the past, considerable attention was paid to the determination of pulmonary artery pressure in COPD patients, but this is now thought to be less important. Undoubtedly, pulmonary artery pressure increases by day and at night47 in hypoxemic COPD patients, reflecting a combination of hypoxic vasoconstriction and pulmonary vascular remodeling. How important this is in the daily limitation of exercise reported by these patients is not clear, but it is known that treatment with domiciliary oxygen prevents disease progression48 and may even reduce pulmonary artery pressure. More specific attempts at therapy, including treatment with vasodilators, phosphodiesterase enzyme type V (PDEV) inhibitors, and nitric oxide—studied inside and outside the ICU—have been unsuccessful, usually resulting in unacceptable worsening of ventilation-perfusion mismatching.49 In general, assessment of pulmonary hypertension has fallen out of favor as part of a routine evaluation in COPD patients, but its occurrence is important to note when interpreting changes in central venous pressure in instrumented patients. Acute rises in pulmonary arterial pressure can follow a pulmonary embolism. Although this can lead to rather atypical COPD exacerbations with persistent hypoxemia,50 this is uncommon in routine practice.51
 Systemic Effects
Systemic Effects
There is good evidence that systemic (extrapulmonary) factors are important in COPD. Patients with a reduced body mass index die sooner than better-nourished individuals with a similar degree of pulmonary function impairment, although those who can gain weight fare better.52 There are data to show that peripheral muscle function is impaired,53 fiber type is altered,54 and exercise is associated with increased oxidative stress.55 The earlier concept of a specific COPD myopathy has now largely been abandoned, as the major burden falls on the lower limb muscles, with preserved function in the upper limb muscle groups. This likely reflects inactivity, which is worse in those with exacerbated COPD.56 Weakness of the quadriceps muscle is an independent guide to a poor prognosis.57 In contrast, the wealth of circulating biomarkers in COPD have contributed little to practical management so far.58
 Exacerbations
Exacerbations
An exacerbation of COPD is currently defined as sustained worsening of the patient’s condition from the stable state, beyond normal day-to-day variation, that is acute in onset and necessitates a change in regular medication.7 The key feature is the sustained change from usual daily symptoms. The operational requirement for a change in treatment is more arbitrary but is almost always present in patients referred for ICU care. Disease exacerbation is the principal cause of ICU admission with COPD, and patients commonly have or are at risk of developing significant respiratory failure, defined as a PaO2 below 60 mm Hg with or without an increase in PaCO2.59 The most common causes of exacerbation are listed in Table 61-1. Viral and bacterial infections are both relevant,54 with rhinoviruses commonly reported in most series; Haemophilus influenzae and Streptococcus pneumoniae are the principal microbial pathogens.60,61 Some patients, particularly those with a regular cough and green sputum production, develop persistent lower respiratory tract colonization, making the interpretation of qualitative microbiology difficult.62 Usually there is an increase in the absolute number of colony-forming units of microorganisms in these patients during exacerbations, reflecting an increased burden of infection, although more subtle changes have been reported involving the introduction of a different serotype of H. influenzae63 without substantial changes in the total bacterial load.64
TABLE 61-1 Causes of Chronic Obstructive Pulmonary Disease Exacerbation
* Clinical presentation is dominated by the primary illness, but respiratory failure can occur.
Not all exacerbations of COPD have an infectious precipitant, and changes in the degree of atmospheric pollution can precipitate events in some patients.65 How frequently individuals develop exacerbations after exposure to a specific precipitating event is not clear, although the likelihood of meeting the consensus definition rises as spirometric impairment worsens.66
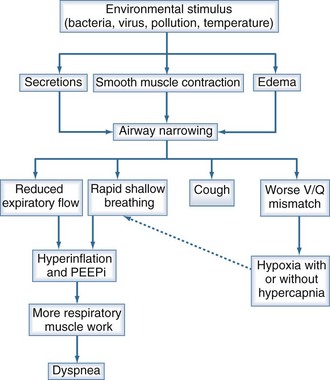
The physiologic consequences of increased airflow obstruction secondary to increased inflammation within the bronchial tree are summarized in Figure 61-2. Whatever the precipitant, the key event appears to be a change in lung mechanics. Previously, attention focused on alterations in respiratory system resistance, but more recent data emphasize that airway narrowing and closure may be more important, particularly by producing changes in operating lung volumes (see earlier discussion). Observations in patients recovering from hospitalized exacerbations have shown progressive improvements in respiratory system reactance (a measure of inspiratory resistance and flow limitation ) together with reductions in end-expiratory lung volume that are most evident in patients reporting less dyspnoea.67 These changes are larger than those in spirometry and help explain why the small changes in FEV1 associated with exacerbations can be associated with substantial deterioration in gas exchange and clinical well-being, leading to hospitalization.
Pneumonia is an important reason for hospitalization in COPD, is more frequently seen in these patients than in others, and is associated with worse outcomes.68 Pneumonia is diagnosed more frequently in patients taking the inhaled corticosteroid, fluticasone propionate,69 especially older patients with worse airflow obstruction.70 These pneumonias are not necessarily associated with poor outcome in terms of mortality or health status69 and are not seen with all types of inhaled corticosteroids.71,72 At present the benefit of inhaled corticosteroid treatment, especially combined with a long-acting inhaled bronchodilator, outweigh the apparent risk of increased pneumonia events.
 Clinical Features
Clinical Features
Key clinical features of the acute presentation of COPD are summarized in Table 61-2. In addition to obtaining an appropriate history and performing a physical examination, with particular attention to the respiratory rate, it is necessary to assess the degree of abnormal gas exchange and the presence of acidosis by measuring arterial blood gases. In the context of an exacerbation, more direct measurements of lung mechanics are usually impractical, and the severity of the mechanical problem is evaluated indirectly by its effect on gas exchange. An urgent chest radiograph is useful for identifying specific precipitating factors, particularly alveolar shadowing due to infection, the presence of a pneumothorax, or radiographic features of pulmonary edema. The last is especially important, because it is commonly associated with hypercapnic respiratory failure, with the combination of an increased ventilatory drive and poor perfusion of respiratory muscles, together with further impairment of ventilation-perfusion matching favoring CO2 retention. In this context, an electrocardiogram is invaluable to screen for both underlying ischemic heart disease and rhythm disturbances. If a major thromboembolic event is suspected on clinical grounds, quantitative D-dimer and urgent computed tomographic pulmonary angiography is the best way to establish this diagnosis. Simple laboratory tests, such as the hemoglobin and white cell count, can be valuable guides to the need for oxygenation and the likelihood of coexisting sepsis.
TABLE 61-2 Clinical Features of Chronic Obstructive Pulmonary Disease Exacerbation
 Intensive Care Unit Referral
Intensive Care Unit Referral
The need for ventilatory support is the primary reason for ICU referral among COPD patients. Although the various indications for mechanical ventilation (Table 61-3) vary in frequency from institution to institution, they represent the most common causes for ICU admission.
TABLE 61-3 Indications for Invasive Mechanical Ventilation
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|