B. First stage of labor. Pain during the first stage of labor is essentially visceral in nature and occurs due to a combination of both uterine contractions and endocervical dilation. Although uterine contractions likely result in myometrial ischemia causing release of bradykinin, serotonin, histamine, and other mediators, mechanoreceptors are also stimulated by stretching and distension of the lower uterine segment and cervix. The noxious stimuli then follow sensory nerve fibers that accompany sympathetic nerve endings, traveling through the paracervical region as well as the pelvic and hypogastric plexi to enter the lumbar sympathetic chain. Visceral nociceptive fibers transmit these impulses to the spinal cord through the posterior nerve roots of T10–L1.
The anatomic basis of pain produced during the first stage of labor implies that the pain is amenable to blockade of peripheral afferents (by epidural block of T10–L1, paracervical block, lumbar sympathetic block) or blockade of spinal cord transmission by intrathecal injection of local anesthetics or opioids.
C. Second stage of labor. Additional painful stimuli are added during the second stage of labor. Beginning with fetal descent, distension and transient ischemia of the vaginal canal, vulva and perineum also occur. These painful somatic impulses are transmitted by the afferent fibers of the pudendal nerve (S2–S4) for subsequent relay in the spinal cord.
The anatomic basis of pain produced during the second stage of labor implies that the pain is amenable to several techniques, including saddle block spinal, pudendal nerve block and/or extension of epidural blockade from T10–S4.
D. Effective blockade in both stages of labor. Pain signals from both the first and second stage of labor are subjected to further processing in the spinal cord, relayed to the supraspinal centers, and ultimately projected to the sensory cortex. Effective blockade of afferent transmission during both stages of labor requires adequate coverage of neuraxial segments T10–S4.
CLINICAL PEARLEffective blockade of afferent transmission during both stages of labor requires adequate coverage of neuraxial segments T10–S4.
II.Neuraxial anatomy in pregnancy
A detailed discussion of lumbar anatomy is beyond the scope of this chapter. Pregnancy, however, is known to induce a number of anatomic changes in the spine. Both anatomic and physiologic changes occur throughout pregnancy and add to the complexity of administering neuraxial analgesia during labor.
A. Reduction in intervertebral gap. The lumbar lordosis is exaggerated due to the parturient’s effort to stabilize her center of gravity. This alteration in lumbar anatomy reduces the dimensions of the intervertebral space making it technically more difficult to perform the procedure.
B. Widening and rotation of the pelvis. Effects of the hormones of pregnancy and the mechanical strain imposed by the fetus result in widening of the pelvis. This has two implications:
1. A head-down tilt results when the parturient is in the lateral position. This can enhance rostral spread of local anesthetics when injected intrathecally.
2. Forward rotation of the pelvis causes the line connecting the iliac crests to cross the spine at a higher level (L3–L4 interspace) compared to nonpregnant patients (L4 body or L4–L5 interspace). This can result in underestimation of the level of needle placement (i.e., L2–L3 instead of L3–L4).
CLINICAL PEARLForward rotation of the pelvis causes the line connecting the iliac crests to cross the spine at a higher level.This can result in underestimation of the level of needle placement (i.e., L2–L3 instead of L3–L4).
C. Higher level of the apex of thoracic kyphosis. The apex of the thoracic kyphosis is shifted to a higher level in pregnancy, from T8–T6, which increases the possibility of increased rostral spread of hyperbaric local anesthetics in the supine position (see Fig. 10.2).
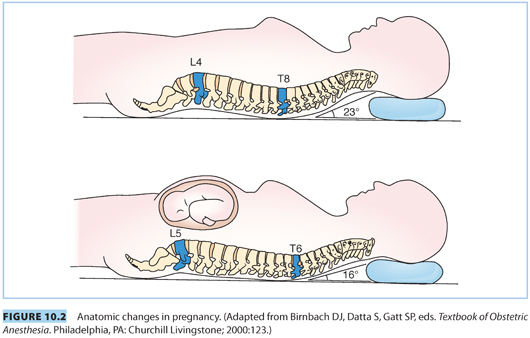
D. Engorgement of epidural veins. Compression of the inferior vena cava and expansion of plasma volume lead to engorgement of epidural veins, increasing the likelihood of vascular cannulation during epidural placement.
E. Difficult identification of the ligamentum flavum. The hormonal changes of pregnancy (i.e., edema) may make the ligamentum flavum difficult to appreciate, increasing the risk of accidental dural puncture.
CLINICAL PEARLThe hormonal changes of pregnancy (i.e., edema) may make the ligamentum flavum difficult to appreciate, increasing the risk of accidental dural puncture.
III.Techniques for neuraxial analgesia during labor
A. Indications
1. Maternal request. The American Society of Anesthesiologists (ASA) and the American College of Obstetricians and Gynecologists (ACOG) have jointly endorsed a statement several times emphasizing that maternal request per se is sufficient indication for analgesia:
a. Labor results in severe pain for many women. In most obstetric patients, the primary indication for epidural analgesia is the patient’s desire for pain relief. There is no other circumstance where it is considered acceptable for a person to experience untreated severe pain, amenable to safe intervention, while under a physician’s care. In the absence of a medical contraindication, maternal request is a sufficient medical indication for pain relief during labor.3 Neuraxial analgesia is widely accepted as the most effective form of labor analgesia.
b. ACOG directly endorses provision of neuraxial analgesia without meeting arbitrary labor progress milestones in this circumstance.
c. “American College of Obstetricians and Gynecologists previously recommended that practitioners delay initiating epidural analgesia in nulliparous women until the cervical dilation reached 4–5 cm. However, more recent studies have shown that epidural analgesia does not increase the risks of cesarean delivery. . . . The fear of unnecessary cesarean delivery should not influence the method of pain relief that women can choose during labor.”4
2. Anticipation of operative delivery, including malpresentation and multiple gestation
3. Obstetric disease (e.g., preeclampsia, nonreassuring fetal heart rate [FHR] tracing) places the patient at high risk for precipitous, high-risk, or emergency delivery.
4. Maternal conditions (e.g., morbid obesity, difficult airway, malignant hyperthermia) complicating or contraindicating general anesthesia
5. Maternal coexisting disease (e.g., severe cardiac or respiratory disease) in which the physiologic consequences of unmitigated pain need to be avoided
CLINICAL PEARLIn most obstetric patients, the primary indication for epidural analgesia is the patient’s desire for pain relief. There is no other circumstance where it is considered acceptable for a person to experience untreated severe pain, amenable to safe intervention, while under a physician’s care.
B. Contraindications (see Table 10.1)
1. Absolute. Patient refusal, uncooperative patient, moderate or severe bleeding diathesis, anticoagulation, uncontrolled hemorrhage and severe hypovolemia, epidural site infection, unskilled or inexperienced anesthesia provider
2. Relative. Elevated intracranial pressure (due to concern about the potential consequences of accidental dural puncture), documented local anesthetic allergy (although alternative drugs may be considered), untreated systemic infection, severe preexisting neurologic deficit, foreign language without adequate interpretation, severe fetal depression, severe maternal cardiac disease (e.g., Eisenmenger syndrome), some skeletal anomalies and following some types of back surgery
C. Rationale for choice of technique
Anesthesiologists may choose any of the three principal techniques (epidural, combined spinal-epidural [CSE], continuous spinal) for a number of reasons: maternal coexisting disease considerations, obstetric considerations, and anesthetic considerations.
1. Maternal coexisting disease
Few maternal conditions clearly favor one neuraxial technique over another. However, a conventional epidural technique may be preferred in these situations:
a. Severe valvular heart disease, uncontrolled hypertension, or other cardiovascular conditions in which central volume changes would be poorly tolerated (e.g., pulmonary hypertension). The slowly titrated onset of an epidural-induced sympathetic block may be physiologically advantageous in such patients.
b. Intracranial mass lesions and other central neurologic diseases (e.g., multiple sclerosis) lead some anesthesiologists to avoid deliberate dural puncture, but the effect of this strategy on outcome has not been demonstrated.
CLINICAL PEARLMaternal coexisting disease, obstetric, and anesthetic considerations will affect the choice of neuraxial technique.
2. Obstetric considerations
a. When surgical intervention is very likely (e.g., nonreassuring fetal status), some anesthesiologists in the past incorrectly favored the epidural technique for labor in order to ensure the epidural catheter was functional in order to avoid general anesthesia. A large retrospective study confirmed that epidurals placed as part of a CSE are more reliable than those placed alone.5 This has been confirmed more recently by a large randomized trial comparing CSE with traditional epidural catheters.6
b. The choice of analgesic method in early labor is controversial, but recent evidence favors the CSE technique over systemic opioids or conventional epidural, citing faster progress of labor as an advantage.7,8 The goal of neuraxial analgesia is to provide effective and safe pain control while improving patient satisfaction and without imposing a negative effect on obstetric outcomes.
c. In active labor, CSE offers somewhat faster onset of analgesia than a conventional epidural. A randomized controlled trial in a private practice setting compared CSE and traditional epidural analgesia in 800 term parturients.6 They found that patients receiving CSE had better pain scores during the first stage of labor and required fewer top-ups by the anesthesiologist, an important consideration when anesthesia manpower is limited. In contrast, a recent Cochrane Systematic Review concluded that there is “little basis for offering CSE over epidurals in labor, with no difference in overall maternal satisfaction despite a slightly faster onset with CSE.”9 Collectively, the effect may be relatively minimal when modern high-volume, low-concentration epidural analgesia is used with an overall advantage of the CSE of no more than 5 to 10 minutes.
d. In advanced labor, particularly in multiparous patients, the CSE technique is often favored due to its rapid onset of sacral analgesia.10
e. Ambulation may be facilitated by the CSE technique. However, very low dose conventional epidurals have been shown to confer similar motor strength and balance; therefore, this advantage is minimal.
f. Preexisting fetal heart abnormalities may influence the anesthesiologist to choose a conventional epidural technique due to the association of intrathecal opioid injections (and the CSE technique) with FHR abnormalities (e.g., fetal bradycardia).11
The issue of FHR patterns before and after CSE and standard epidural techniques has been addressed in two recent publications.12,13
CLINICAL PEARLIn active labor, CSE offers faster onset of analgesia than a conventional epidural. However, this difference is less when high-volume, low-concentration epidural analgesia is used.
3. Anesthetic considerations
a. Anticipated difficult airway may encourage early placement of an indwelling epidural catheter. Poorly functioning catheters should be replaced early during labor and not waiting until the emergency cesarean delivery (CD) has been called.
b. Early (i.e., before onset of labor or maternal request) insertion of a spinal or epidural catheter for obstetric (e.g., twin gestation or preeclampsia) or anesthetic indications (e.g., anticipated difficult airway or obesity) may be advantageous to reduce the need for general anesthesia if an emergent procedure becomes necessary.14 In addition, cases of impending coagulopathy and decreasing platelet counts (e.g., hemolysis, elevated liver enzymes, low platelets [HELLP] syndrome) may warrant early placement.
c. The rate of postdural puncture headache (PDPH) with CSE is low, approximately 1% or less with modern pencil-point needles. The overall risk of dural puncture with an epidural needle may be reduced by the CSE technique.5
d. Deliberate continuous spinal techniques are rarely used due to the increased risk of PDPH. However, in extreme morbid obesity and selected other situations in which slow-onset surgical anesthesia (e.g., aortic stenosis) is desired, this technique may be appropriate. The inherent dangers of having a woman labor with an indwelling intrathecal catheter limit the use of this practice in many hospitals. These dangers include mistakingly injecting a dose of local anesthetic appropriate for an epidural into the intrathecal space with the risk of high block leading to respiratory arrest and cardiovascular collapse. These catheters need to be well labeled and all care providers informed that the catheter is intrathecal.
CLINICAL PEARLEarly (i.e., before onset of labor or maternal request) insertion of a spinal or epidural catheter for obstetric (e.g., twin gestation or preeclampsia) or anesthetic indications (e.g., anticipated difficult airway or obesity) may be advantageous to reduce the need for general anesthesia if an emergent procedure becomes necessary. Intrathecal catheters should be well labeled and all caregivers informed.
D. Preparation (see Table 10.2)

Adequate preparation is the single most important determinant of success with neuraxial techniques. The ASA’s recommendations for neuraxial anesthesia in obstetrics are outlined in Chapter 33.14
1. Evaluation and consent. A focused history including all relevant obstetric and anesthetic issues, a relevant physical examination (i.e., airway, heart, and lungs, consistent with the ASA “Practice Advisory on Preanesthesia Evaluation”; http://anesthesiology.pubs.asahq.org/article.aspx?articleid=1933628), and determination of the timing of last intake of solids are mandatory. One recent study demonstrates that there are significant airway changes in women during labor and delivery and that it is prudent to reexamine the airway just before initiation of anesthesia during the intrapartum or immediate postpartum period.15 The advantages, potential side effects, and complications (e.g., infection, PDPH, neurologic injury) of neuraxial techniques need to be specifically discussed before obtaining the parturient’s signed consent. Initiating a discussion with the obstetrician is useful in formulating a final plan of care. The ASA practice guidelines reinforce the importance of communication between obstetrician and anesthesiologist. Specifically, “recognition of significant anesthetic or obstetric risk factors should encourage consultation between the obstetrician and the anesthesiologist.”14 It also gives an opportunity for the anesthesiologist to establish rapport with the labor and delivery nurse, an important resource in the obstetric suite.
2. Aspiration prophylaxis. Restriction of oral intake to only clear fluids is an intuitive precaution following neuraxial blockade. However, the restriction of oral intake in labor is controversial, and little evidence supports either very restrictive or very liberal intake rules in the presence of functional neuraxial block. The revised ASA guidelines for obstetric anesthesia reflect this uncertainty, although the panel recommends no intake of solid food during active labor.14 Patients with additional risk factors for aspiration (e.g., morbid obesity, diabetes, difficult airway) or patients at risk for operative delivery (e.g., nonreassuring FHR pattern) may have further restrictions of oral intake, determined on a case-by-case basis.
3. Intrapartum platelet counts and blood type and screen. The anesthesiologist’s decision to obtain a platelet count should be individualized based on a patient’s history, physical examination, and clinical signs. A routine platelet count is unnecessary in healthy parturients. A routine cross match is unnecessary for uncomplicated parturients undergoing vaginal or operative delivery. The decision to order or require a blood type and screen or cross match should be based on maternal history, anticipated hemorrhagic complications, and local institutional policies.
4. Intravenous access. A good, functioning intravenous catheter is required prior to epidural placement. Preloading or coloading with IV fluids can be done according to the prevailing department guidelines. However, a specified volume is unnecessary and will not reliably prevent hypotension.
5. Monitoring. Ensuring maternal and fetal well-being before, during, and after epidural techniques is paramount to successful management of laboring parturients.
a. Noninvasive blood pressure monitoring
Documentation of baseline noninvasive blood pressure (NIBP) upon admission and immediately before epidural placement is recommended. After administration of the bolus dose, NIBP should be measured at least every 5 minutes for up to 20 minutes or until hemodynamic stability is demonstrated. Periodic measurement of NIBP is recommended for the duration of neuraxial analgesia, although the optimal frequency of measurement has not been established.
b. Electronic fetal monitoring. It is necessary to monitor baseline FHR and identify FHR abnormalities during or immediately after placement. The ASA Task Force recommends monitoring before and after initiation of neuraxial analgesia but is equivocal on the need for monitoring during the procedure itself and for continuous monitoring thereafter.14
c. Other monitors. Electrocardiography and pulse oximetry are not usually required. However, a pulse oximeter can provide valuable information regarding maternal heart rate during initiation of epidural analgesia and test dosing.
6. Positioning. Although both sitting and lateral positions are suitable for epidural techniques, the ultimate decision depends on the anesthesiologist’s preference. The advantages of the sitting position include better perception of the midline and increased maternal comfort. The lateral position may, however, seem to have minimal effects on uteroplacental perfusion and appears more suitable for FHR monitoring. Some evidence supports fewer intravascular catheter placements in the lateral position, during which the valveless epidural veins are decompressed.16 However, for those who use the sitting position routinely, intravascular catheter placement is not a common event and is more often influenced by the type of epidural catheter used.
7. Resuscitation drugs and equipment. Immediate availability of resuscitation drugs, vasopressors such as ephedrine and phenylephrine, airway equipment, and supplies are necessary to administer a safe epidural anesthetic and manage complications (e.g., hypotension, systemic toxicity, high spinal anesthesia, respiratory depression). The anesthesiologist must always be prepared to manage these complications.
8. The Joint Commission 2015 National Patient Safety Goals. The name, date of birth, and the proposed neuraxial technique need to be verified with the parturient prior to initiation of the procedure in accordance with The Joint Commission recommendations for patient safety (http://www.jointcommission.org/assets/1/6/2015_HAP_NPSG_ER.pdf; date accessed: April 30, 2015).
CLINICAL PEARLAdequate preparation and optimal positioning are important determinants of success with neuraxial techniques.
E. Description of technique (see Table 10.3)
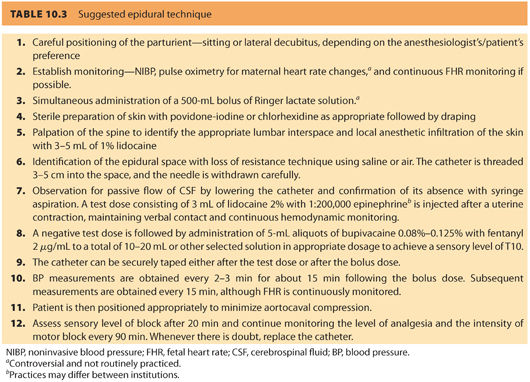
1. Epidural technique
a. The epidural tray. A commercial or self-prepared kit should contain all materials needed for the block. Most commercially available epidural kits contain a 17-G epidural needle (either Tuohy or Weiss), a 19-G or 20-G epidural catheter and ampules of saline and local anesthetics (usually lidocaine 1% and lidocaine 1.5% with epinephrine 1:200,000 for local infiltration and use as a test dose, respectively), skin prep solutions (povidone-iodine or chlorhexidine), sterile drape, gauze wipes, and transparent dressing.
b. Sterile precautions. Hand washing, jewelry removal, and the use of sterile gloves (to supplement hand washing, and not as a substitute) are important sterile precautions, fresh mask, and cap have been shown to reduce the incidence of microbial contamination. Basic components of aseptic technique are often breached. The American Society of Regional Anesthesia (ASRA) consensus statement17 (see Table 10.4) and ASA Taskforce in Obstetric Anesthesia14 acknowledge the importance of these procedures but remain equivocal regarding the use of gowns. Alcohol-based chlorhexidine solution has been convincingly shown to provide superior germicidal properties compared to povidone-iodine (Betadine) skin preparation.17 Although it has been shown that chlorhexidine solutions have faster and stronger bactericidal effects than povidone-iodine, chlorhexidine is not U.S. Food and Drug Administration (FDA)-approved for use as a disinfectant solution before neuraxial techniques due to controversy about neurotoxicity in animal models; nevertheless, this agent is widely used for skin preparation before neuraxial anesthesia.
c. Identification of the lumbar interspace. The ideal lumbar interspaces for labor analgesia are usually L3–L4 or L2–L3. Traditionally, the line connecting the iliac crests is thought to cross the L4 vertebral body or the L4–L5 interspace. It must be realized that identification of such interspaces without radiologic guidance is at best a “guesstimate” and is fraught with error.18,19 In patients with difficult anatomic landmarks, such as the morbidly obese, it is better to use the lowermost lumbar interspace that can be easily located because of risk of underestimating the interspace level. Although not demonstrated in randomized trials, it appears that it is more difficult to approach the epidural space at the L4–L5 interspace than at higher spaces.
Prepuncture ultrasonography may be useful to facilitate epidural placement. Especially in obese parturients, the distance to the epidural space is often underestimated because of compression of the subcutaneous tissue that is often required to compensate for poor visibility. A recent study evaluated scanning in the paramedian sagittal oblique (PSO) plane compared with the transverse median (TM) plane to determine whether scanning in the PSO plane resulted in a more precise estimate of the skin to epidural space measurement.20 The authors concluded that the estimates were comparable with PSO versus TM and that the ability to use both estimates interchangeably may prove useful in patients with poor visibility in the TM plane.
After sterile preparation and draping, a liberal local anesthetic infiltration (3 to 5 mL of 1% lidocaine) is performed over the chosen interspace. The skin is then punctured with the epidural needle and advanced until it is firmly seated in the interspinous ligament. The stylet is removed and the loss of resistance (LOR) syringe is attached to the hub of the epidural needle.
CLINICAL PEARLAlthough not demonstrated in randomized trials, it appears that it is more difficult to approach the epidural space at the L4–L5 interspace than at higher spaces. Prepuncture ultrasonography may be useful to facilitate epidural placement.
d. Techniques
(1) Loss of resistance. This commonly employed method to identify the lumbar epidural space is based on the tactile sensation of a sudden “give way” when the soft tissue resistance offered by the ligamentum flavum is overcome when the needle tip enters the epidural space. A specially designed plastic or glass LOR syringe, customized to reduce frictional and resistive forces between the syringe and the plunger, is attached to the hub of the epidural needle.
(a) Air versus saline. Although the anesthesiologist’s preference may assume primary importance, increasing evidence points to the superiority of saline-based techniques (saline alone, saline with a bubble of air) over air for location of the epidural space.21 The cited advantages include better sensory appreciation, decreased incidence of patchy blocks, and elimination of the risks of epidural venous air embolism and pneumocephalus headache (should an accidental dural puncture occur). Some practitioners use air alone for the CSE technique so that any clear fluid coming back through the spinal needle can only be cerebrospinal fluid (CSF); and not a depot of saline or local anesthetic in the epidural space). If using air, it is important not to empty the syringe into the epidural space after LOR.
(b) Intermittent versus continuous pressure. Application of “intermittent” or “continuous” pressure to the syringe plunger is an integral component of the LOR technique (see Fig. 10.3). Purported advantages of the continuous technique include faster identification of the epidural space and possible reduced incidence of accidental dural puncture. Purported advantages of the intermittent technique include more control over needle advancement (two hands on the needle) and also reduced risk of dural puncture.
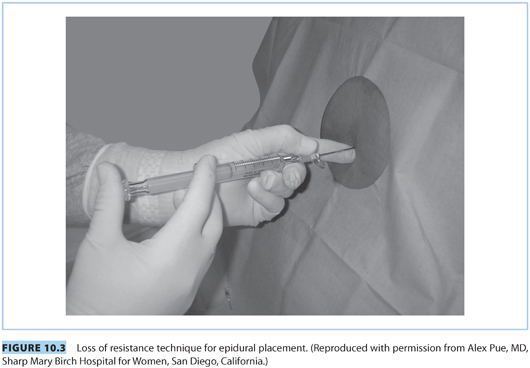
The Episure (see Fig. 10.4) is a spring-loaded syringe that uses saline and is designed to automatically collapse upon entering the epidural space. It offers the advantage of using two hands on the needle with continuous application of plunger pressure. A recent study of experienced obstetric anesthesiologists determined that the Episure syringe for LOR was associated with a similar overall rate for establishing successful epidural labor analgesia and a shorter elapsed time to epidural catheter insertion.22

(2) Bevel orientation. It is common practice to orient the bevel of the epidural needle cephalad. It may help direct the catheter to its intended location (i.e., in the vicinity of the T10–L2 segments). Insertion of the needle with the bevel parallel to the long axis of the back to reduce the risk of PDPH if dural puncture occurs and rotating the needle within the epidural space is not recommended. This maneuver is associated with an increased risk of dural puncture.23
e. The epidural catheter
(1) Material. Most catheters are made of polyamide (nylon). Most catheters are clearly marked in 1-cm increments over the distal 20 cm. Wire-reinforced epidural catheters are more flexible and have been shown to reduce the incidence of paresthesias and intravascular placement in obstetric patients compared to use of more rigid catheters.24
(2) Multihole versus single hole. There is limited evidence demonstrating the superiority of multiport catheters over uniport catheters. Theoretically, multiport catheters may be expected to provide better quality of analgesia due to a more uniform spread of local anesthetic, especially after bolus administration.25 This advantage may be negated by the current use of large-volume, low-concentration mixtures for labor analgesia. In addition, inclusion of opioids in the mixture may limit the theoretical advantage even further, by providing excellent quality segmental analgesia. Multicompartment blocks (e.g., epidural, subdural, intrathecal, and intravascular) have rarely been reported with use of multiport catheters.
(3) Depth of catheter insertion. Increasing the depth of the catheter (≥6 cm) in the epidural space increases the incidence of coiling and unsatisfactory (i.e., unilateral) analgesia, whereas having a reduced length (≤4 cm) increases the likelihood of catheter dislodgement. Overall, inserting the catheter to a depth of 4 to 5 cm appears to be optimal.26
In some cases, blood is noted in the catheter during insertion or patients develop a persistent paresthesia during insertion. When persistent paresthesias occur, the catheter must be removed and replaced. However, the catheter and epidural needle must be withdrawn simultaneously. Otherwise, withdrawing the catheter through the epidural needle risks shearing of the catheter. If this occurs and the broken catheter remains in the patient’s back, the patient should be informed and a neurosurgeon consulted. However, surgical removal is rarely needed unless there are persistent neurologic symptoms.
If an IV catheter is detected on aspiration, the catheter can be withdrawn 0.5 to 1 cm at the time until blood is no longer aspirated. If the catheter tip remains at an adequate depth and is no longer in the vein, the catheter should be tested and secured. This technique may salvage up to 50% of these catheters. However, in some cases, the depth will be inadequate and the catheter should be replaced.
CLINICAL PEARLWhen persistent paresthesias occur, the epidural catheter must be removed and replaced.
f. If a test dose is used, it is recommended to administer it before securing the catheter. The test dose will be discussed at depth in a subsequent subsection of the chapter.
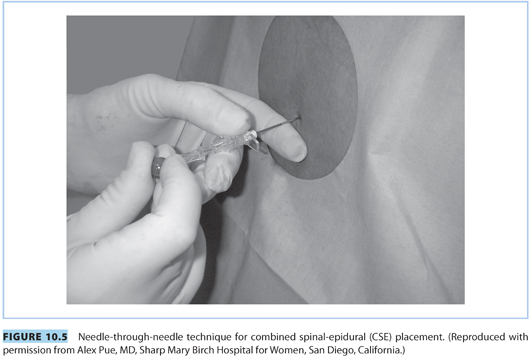
2. Combined spinal-epidural technique
The CSE and conventional epidural techniques are similar, with a few exceptions. The technique incorporates the beneficial effects of intrathecal analgesia (i.e., rapid onset, better quality, and increased sacral spread) to the flexibility offered by an epidural catheter.
a. “Needle-through-needle” technique (see Fig. 10.5). The procedure for locating the epidural space is the same as described previously. Once the 17-G epidural needle is in the epidural space, a 25- to 27-G pencil-point spinal needle is inserted through the epidural needle and advanced until a distinct “pop” is appreciated and CSF is visualized in the spinal needle. The spinal needle is stabilized and the intrathecal dose of local anesthetic (e.g., bupivacaine 1.5 to 3 mg) and/or opioid (e.g., fentanyl 5 to 15 μg) is administered when CSF appears at the hub.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







