
Chest Pain
Judd E. Hollander
Chest pain is the second most common chief complaint in patients presenting to the emergency department (ED). However, when combined with other symptoms that potentially represent myocardial ischemia, pulmonary embolism (PE), or aortic dissection (such as back pain, neck pain, shortness of breath, etc.), the evaluation of patients with “chest pain equivalents” is the most commonly evaluated diagnostic entity.
The evaluation of chest pain is complicated by the fact that the differential diagnosis includes several life-threatening entities that must be rapidly diagnosed (acute myocardial infarction [AMI], aortic dissection, PE) while recognizing that the majority of patients with chest pain have less urgent conditions. The emergency medicine approach to determining the etiology of chest pain cannot focus solely on what is more likely and what is less likely. It must focus on reducing the likelihood of the serious causes of chest pain to an acceptable threshold. This is usually considered to be less than a 1% to 2% likelihood of missing the diagnosis or having 30-day adverse events (1–3).
CLINICAL PRESENTATION
Chest pain, shortness of breath, and other symptoms of potential acute coronary syndrome present an initial diagnostic challenge. The cardiac, pulmonary, gastrointestinal, abdominal, and musculoskeletal systems all share somatic or visceral nerve roots, making it difficult to accurately distinguish the etiology of chest pain based upon history and physical examination. Demographic characteristics, traditional risk factors, chest pain characteristics, and physical examination are insufficiently accurate to exclude life-threatening diagnoses by themselves (4,5), but they can help direct appropriate diagnostic testing. Unfortunately, there is no sufficient availability of diagnostic tests for acute coronary syndrome with enough sensitivity to confidently rule out the condition rapidly in the ED. Therefore, after a thorough evaluation, the clinician is often left admitting or observing many patients to rule out acute coronary syndrome. Patients with AMI can have a wide variety of symptoms, making diagnosis difficult—in fact, atypical symptoms are so common that up to 40% of non–ST-segment elevation AMIs go unrecognized by the patient (6).
Classically, the pain of an AMI or acute coronary syndrome is substernal or left chest pressure or aching and may have radiation to the arm or neck. High-risk features of AMI, such as left arm radiation, substernal location, and past history of AMI are reliable with good interrater reliability and can be used to predict increased risk of AMI. On the other hand, low-risk features such as pleuritic, positional, and sharp chest pain have poor reliability making it difficult to rule out ACS (4). Traditional cardiac risk factors, such as hypertension, family history of heart disease at a young age, diabetes, and increased cholesterol help predict development of coronary disease over decades, but the absence of them is not useful to risk stratify patients in the ED (5).
Aortic dissection classically presents with a severe anterior tearing pain that may radiate to the back between the shoulders. The pain can be perceived as migratory, as the dissection extends. Patients often appear sick and may be writhing in pain. A pulse differential is actually very uncommon and lack of one should not be considered to exclude the condition.
A prominently pleuritic pain with or without shortness of breath can be caused by pleural inflammation from PE, pneumonia, pleurisy, and pericardial inflammation (from pericarditis or myocarditis). Pericarditis may be positional and is classically worse with lying down but the pericardial rub is easier to hear when patients are sitting up. Pneumothorax is usually sudden onset and worse with breathing. Fever and purulent cough suggest pneumonia.
Esophageal spasm, gastroesophageal reflux disease, and other GI causes of chest pain can be difficult to diagnose in the ED. They can have pain similar to ACS in terms of quality, location, radiation, and intensity and can also be associated with ill appearance and diaphoresis, although shortness of breath is less common. Cholecystitis and biliary colic are likely to have right upper quadrant or midepigastric tenderness and as long as complete physical examination is performed, they should be able to be diagnosed.
The ability to reproduce pain by pushing on the chest wall is likely to be misleading. Although musculoskeletal causes of chest pain are common, approximately 6% of patients diagnosed with costochondritis are actually having an AMI (7). Undressing the patient and examining their skin should identify most cases of herpes zoster, although patients can have burning dermatomal pain for a couple of days before the rash becomes evident.
Patients may improve with either a “GI cocktail” or with nitroglycerin whether or not their symptoms were from a GI or cardiac etiology. Clinicians should not rely on response to treatment in determining the etiology of pain (8,9).
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of chest pain is large. Although often broken down into the organ system involved (cardiac, pulmonary, gastrointestinal, abdominal, or musculoskeletal), it is better broken down into diagnostic categories in terms of levels of urgency. The treatment of each of these conditions is not covered in this chapter, but can be found in the chapters on each specific disease.
Critically time-sensitive conditions are those that can worsen rapidly if left untreated or can benefit from time-sensitive surgical or medical interventions. These include Type A and B aortic dissection, ST-segment elevation myocardial infarction (STEMI), hemodynamically significant PE, tension pneumothorax, pericardial tamponade, esophageal perforation, and mediastinitis. All these conditions have interventions that can improve outcome, if the diagnosis is made in a timely manner.
Urgent or emergent diagnoses to consider before ED disposition include acute coronary syndromes (non–ST-segment elevation myocardial infarction [NSTEMI] or unstable angina), submassive (nonhemodynamically significant) PE, pneumonia, pneumothorax, pericardial effusion, pericarditis, cholecystitis, pancreatitis, and Fitz-Hugh–Curtis. Significant delays in treatment of patients with these conditions can result in adverse outcomes.
Chest pain can result from cardiac conditions where myocardial demand outstrips myocardial supply, or myocardial injury occurs even without coronary artery disease. Such conditions include cocaine- or amphetamine-induced ischemia, hypertropic cardiomyopathy, myocarditis or valvular heart disease (aortic stenosis in particular).
Less urgent conditions that may still cause significant discomfort to patients include intercostal strain, costochondritis, rib pain, radicular pain from cervical or thoracic spine disease, and herpes zoster or postherpetic neuralgia all of which may be radicular in nature. Gastrointestinal conditions such as gastroesophageal reflux disease or peptic ulcer disease may be suggested by symptoms. Pleurisy or epidemic pleurodynia can mimic more severe conditions. Mitral valve prolapse is associated with chest pain, the etiology of which is unclear. Fibromyalgia and psychiatric causes of chest pain should also be considered and they can be difficult to distinguish from more serious causes of chest pain.
DIAGNOSTIC APPROACH
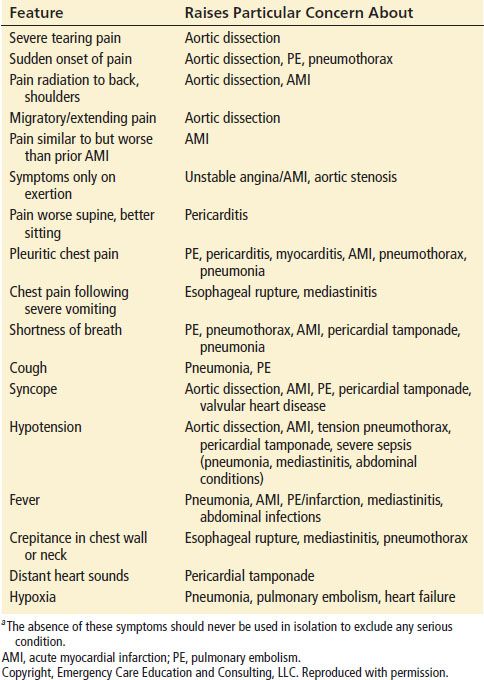
Since the differential diagnosis of chest pain is broad and the history and physical examination often lack sufficient accuracy to exclude the most serious conditions, the diagnostic approach must take into account the pretest probability of these serious conditions while performing diagnostic testing to reduce the posttest probability of missing one of the serious causes to less than 1% to 2%. Clinical features particularly concerning for high-risk conditions are noted in Table 6.1. The absence of any of these features should not be used to exclude the possibility of these conditions. More detailed descriptions of the classic presentations of each condition can be found in the chapter specific to that disease.
TABLE 6.1
Selected Clinical Features that Raise Particular Concern for Serious Conditions in Patients with Chest Paina
12-Lead Electrocardiogram
The diagnostic approach should begin with a 12-lead electrocardiogram (ECG). Ideally the ECG should be accomplished within 10 minutes of arrival and is often done in conjunction with triage and before or concurrent with the history and physical examination. The 12-lead ECG will identify STEMI and allow STEMI protocols to be activated in a timely manner (see chapter on AMI). ST-segment depressions or T-wave inversions may identify patients with non–ST-segment elevation acute coronary syndromes. PR depression or diffuse ST-segment elevations can suggest pericarditis or myocarditis. P pulmonale, right axis deviation, right ventricular strain pattern, right bundle branch block, or an S1Q3T3 suggest PE. Electrical alternans suggests pericardial tamponade. Unfortunately, a normal ECG cannot exclude any of these conditions—it is normal in 5% and nondiagnostic in as many as 50% of patients with AMI. Similarly, it is normal or nondiagnostic in up to 85% of patients with aortic dissection.
If the ECG does not show STEMI, further diagnostic testing is usually dictated by the history, physical examination, and results of chest radiography.
Chest Radiography
Most but not all chest pain patients will receive chest radiography. The diagnosis of pneumonia, pneumothorax, esophageal perforation, mediastinal emphysema, free air under the diaphragm, and pleural effusion are easily made.
Most patients with ACS will have nondiagnostic chest radiography. A widened mediastinum raises concerns regarding aortic dissection (10). A widened aortic knob or widened mediastinum increases the odds of aortic dissection 11-fold. It can be found in up to 76% of such patients. Aortic displacement and pleural effusions are other findings that can be associated with aortic dissection.
Although most patients with PE will not have pathognomonic findings on chest radiography, a Hampton hump (wedge-shaped pleural defect), Westermark sign (decreased vascularity distal to the occlusion) can suggest its diagnosis. More commonly seen are nonspecific findings such as atelectasis, pleural effusion, or elevated hemidiaphragm.
After consideration of the history, physical examination, ECG, and chest radiograph further diagnostic testing is usually dictated based upon likelihood of each item remaining in the differential diagnosis.
Cardiac Troponins
Most patients with chest pain will receive laboratory testing for cardiac troponin. Cardiac troponin elevation is the gold standard for the diagnosis of AMI, although it can also be elevated in conditions of myocardial injury unrelated to thrombotic occlusion. Current ACC/AHA guidelines still advise serial troponin values for 8 to 12 hours or at least one cardiac troponin 8 to 9 hours after chest pain onset (11).
High-sensitivity cardiac troponin, which is expected to be on the market in the US in late 2013, has approximately 90% sensitivity and 80% to 85% specificity for the diagnosis of AMI at the time of ED arrival and the sensitivity rises to nearly 100% within 3 hours of arrival (12–14). This will allow more rapid “rule outs” than contemporary assays (15).
Regardless of the etiology of the troponin elevation, more troponin is always worse than less troponin. By definition, high-sensitivity troponins will result in measurable troponin values in 50% of the health population. Measurable amounts of troponin, even though below the upper limit of normal conventional assays (for example, still below 0.04) portend a worse prognosis than when below the level of detection. Troponin is associated with a worse prognosis in AMI, heart failure, renal failure, sepsis, GI bleeds, cocaine use, and asymptomatic volunteers.
High troponin elevations are most likely from AMI, but other etiologies of chest pain such as PE can have low-grade troponin elevations. The use of delta troponins (two troponins 2 hours apart) can determine whether the troponin is rising, in which case it is most likely due to myocardial ischemia, or is stable, in which case it is usually due to some other etiology. The precise delta value is assay and patient population dependent, so the EP should know the test performance characteristics at his or her own institution.
D-Dimer
D-dimer is a relatively nonspecific test that can be useful to assist in the diagnosis of PE and aortic dissection. In patients with a low pretest probability for PE, a negative D-dimer can obviate the need for further diagnostic testing. D-dimer is a nonspecific test, and is often elevated in the absence of PE in patients who are elderly, have recent surgery, malignancy, trauma, or infections. Therefore, it should not be ordered indiscriminately and when ordered routinely at triage, should not mandate further testing for PE. Pretest probability assessment and pulmonary embolism “rule out” criteria testing should be used to determine whether patients need further noninvasive testing for PE (3) (see Pulmonary Embolism chapter). A negative D-dimer reduces the likelihood of aortic dissection dramatically, as the LR is 0.07 (16). It may be used to rule out the diagnosis in low-risk patients.
Computed Tomography
CT scans can help identify pneumothorax, pericardial effusion, mediastinitis, esophageal perforation, pneumonia, and abdominal causes of chest pain. CT angiography can be used to diagnose aortic dissection, coronary artery disease and myocardial ischemia, and PE. The timing of contrast injection, amount of contrast, and imaging protocol differ depending upon whether the CT is being done for evaluation of PE, aortic dissection, or coronary anatomy. There are three randomized controlled trials demonstrating that coronary CTA is safe, effective, more efficient, and cost-effective for the evaluation of low- to intermediate-risk patients with potential acute coronary syndromes and nondiagnostic ECGs. There was a less than 1% event rate in patients with normal coronary CTA over the ensuing 30 days, 6 months, and 1 year, and coronary CTA resulted in a shorter length of stay and higher ED discharge rate (17–19).
Some advocate “triple rule-out” scans where the contrast bolus is timed to provide diagnostic information about all three of those serious conditions. There are comparisons of triple rule-out scans with dedicated coronary CTA but there have not been comparative studies evaluating diagnostic utility of triple rule-out scans with dedicated PE and aortic dissection scans (20).
Bedside Ultrasound
Bedside ultrasound can be used to identify intra-abdominal catastrophes (abdominal aortic aneurysm/rupture), cholecystitis, biliary colic, pneumothorax, pleural effusion, pericardial tamponade and pericardial effusions, cardiac wall motion abnormalities suggestive of myocardial ischemia, ventricular dysfunction, and valvular heart disease. It cannot, however, distinguish old from new wall motion abnormalities and cannot be used to “rule out” an acute coronary syndrome, since it misses approximately 6% of NSTEMI.
Myocardial Perfusion Imaging
Resting sestamibi imaging can identify myocardial ischemia at the time of presentation. Technetium sestamibi is distributed within the myocardium in proportion to myocardial blood flow and it has prolonged retention with minimal redistribution. A normal scan identifies patients with a very low likelihood of AMI or cardiac death (none of 338 patients) over the ensuing year (21). The ERASE trial found that, when compared to the typical ED evaluation (cardiac markers and ECG), the use of rest sestamibi scans resulted in similar diagnostic ability but a higher rate of ED discharges (22).
Stress myocardial perfusion imaging or stress echocardiography is common postacute care and often used in observation units to exclude exercise-induced ischemia. Patients with an uneventful observation period, negative cardiac markers, and a normal stress test can be safely discharged with a referral for follow-up. There is evidence supporting the use of immediate exercise testing in low-risk ED chest pain patients without known ventricular dysfunction and with normal or nonspecific ECGs, even without serial cardiac marker determination (23,24).
CRITICAL INTERVENTIONS
• Obtain an ECG within 10 minutes of arrival.
• Obtain cardiac troponin results within an hour of arrival.
• Obtain chest radiography to help identify many possible causes of chest pain.
• Treat tension pneumothorax clinically without waiting for radiographic imaging.
• Do not forget to assess skin, chest wall, and abdominal causes of chest pain.
• Treat vital signs depending upon differential diagnosis. Some conditions (aortic dissection) require more aggressive treatment of blood pressure and heart rate.
• Safely reduce pain, regardless of etiology. Since response to nitrates and GI cocktail do not help you discern pain etiology, some patients can be given analgesics, nitrates, and a GI cocktail to help make them comfortable, regardless of the cause of the pain.









