
Asthma
Lauren M. Smith
Asthma is characterized by increased airway responsiveness to various stimuli. Asthma is caused by a variety of processes including bronchoconstriction, widespread bronchial wall edema, and increased mucous secretions. However, airway inflammation is the primary pathophysiologic mechanism. Although the reversibility of airflow obstruction was once considered a cornerstone of the diagnosis, it is now recognized that reversibility may be incomplete in some patients because of airway remodeling (1).
Asthma affects between 5% and 10% of adults in the United States and the prevalence of asthma has been on the rise for the last three decades. However, mortality has declined. Asthma accounts for just over 2 million emergency department (ED) visits per year, adult and pediatric. Most deaths occur prior to hospital arrival and the highest rates of death occur in those older than 65. About half of all cases are diagnosed before the patient reaches age 10, and another one-third are detected before age 40. The male-to-female ratio of this disease is higher during childhood but reverses in adulthood. Higher asthma prevalence is seen in lower socioeconomic households and in the Afro-American population (2).
The natural course of asthma has not been investigated thoroughly, but studies suggest that 50% to 80% of patients have a good prognosis, especially those with mild disease or disease that develops in childhood. Chronic asthma is categorized by severity, ranging from intermittent to mild, moderate, or severe persistent. Treatment is focused on reduction of impairment and reduction of risk. Periods of symptom-free intervals are interspersed with exacerbations, which trigger visits to the ED. Only about l0% or less of asthmatics are considered to have severe or refractory asthma, and it is these patients that are difficult to control and are prone to exacerbations.
Asthma is a heterogeneous disease. Clinically, it can be separated into two groups: allergic or extrinsic asthma, and nonallergic or intrinsic asthma. Many patients have components of both types. Extrinsic asthma accounts for <10% of patients with asthma and tends to develop early in life. It is associated with a well-defined sensitivity to inhaled allergens, a family history of allergic diseases, increased serum levels of immunoglobulin (Ig) E, positive immediate skin tests, and blood eosinophilia. It may be seasonal (trees, grasses, and spores) or perennial (animals, gardens, and house dust). A good response to bronchodilator therapy is usually observed.
Intrinsic asthma is the more common type of the disease. An inciting allergen cannot be identified, a family history of allergies is less common, IgE levels may be normal or low, and skin tests are negative. Intrinsic asthma is perennial, tends to be more severe than extrinsic asthma, and has a limited response to bronchodilator therapy.
All individuals with asthma have hyperresponsive airways that narrow when exposed to various stimuli: allergic, infectious, pharmacologic, environmental, occupational, exercise-related, or emotional stimuli.
Allergic asthma occurs when inhaled allergens bind to IgE molecules that are bound to mast cells in the lining of the tracheobronchial tree. During the early response, various mediators are released, causing increased vascular permeability, mucosal edema, and bronchial smooth-muscle contraction. A second wave of reaction, the late response, is seen hours to days later and involves the accumulation of inflammatory cells in the bronchial mucosa, thereby perpetuating the reaction.
Although several theories attempt to explain the pathophysiologic changes that occur in nonallergic asthma, none adequately explains all clinically observed phenomena. Research suggests that even patients without atopy have the same pathophysiologic basis for the disease as atopic patients.
Respiratory infections, particularly viral infections, commonly precipitate bronchospasm. Viruses cause mucosal inflammation and lower the firing threshold of the subendothelial vagal receptors, resulting in enhanced airway reactivity. This hyperactivity may last up to 8 weeks, even in nonasthmatic persons.
Pharmacologic agents also may induce acute asthma. The agents most frequently implicated are aspirin and nonsteroidal anti-inflammatory compounds, coloring agents, and β-adrenergic antagonists.
Up to 10% of adults with asthma experience the triad of aspirin-induced bronchospasm, nasal polyps, and eosinophilia. Ingestion of aspirin, nonsteroidal anti-inflammatory compounds, and tartrazine (FDC yellow dye no. 5) and other dyes may induce severe asthma in these patients, possibly by diverting arachidonic acid metabolism toward the lipoxygenase pathway and causing the production of leukotrienes, which are potent bronchoconstrictors.
Sulfating agents are used widely as food preservatives and antioxidants in pharmaceutical products. Exacerbation of asthma has been reported after food ingestion and after the use of sulfite-containing drugs, including some inhalation bronchodilators.
A large variety of occupational dusts and fumes may provoke acute airway obstruction. Patients with occupational asthma typically give a cyclic history. They are symptom-free during weekends, vacations, and on arrival at work. As the workday progresses, wheezing develops. A history of similar symptoms in fellow employees may be noted. Three mechanisms are involved in occupational asthma: immunologic reactions (e.g., animal handlers), direct liberation of bronchoconstrictors (e.g., cotton workers), and direct irritant effects (e.g., meat wrappers).
Exercise also may stimulate an asthma attack. Exercise-induced bronchospasm is usually noted within 5 to 20 minutes after the completion of exercise and is related to thermal changes in the respiratory tree. Exercising in a cold, dry environment causes a more marked response than exercising in a warm, humid environment.
Endocrine factors have been recognized as yet another cause of airway hyperresponsiveness. Normal variations in progesterone and estradiol levels are believed to modulate airway reactivity.
Psychological factors also influence asthma exacerbations, probably through modification of vagal efferent activity. The extent to which psychological factors participate in the induction and continuation of an asthma attack is unknown but probably varies from patient to patient and from episode to episode.
Regardless of the underlying precipitant, airway narrowing, bronchial wall edema, bronchial smooth muscle contraction, and mucosal plugging ensue. These changes result in increased airway resistance, decreased forced expiratory volumes and flow rates, lung hyperinflation, increased work of breathing, and ventilation–perfusion mismatch.
CLINICAL PRESENTATION
The classic triad of symptoms during an asthma attack is cough, dyspnea, and wheezing, but chest tightness and nonproductive cough may precede dyspnea and wheezing. Asthmatic patients coming to the ED are frequently anxious, tachypneic, tachycardic, and mildly hypertensive. As airway obstruction worsens, the expiratory phase becomes prolonged and lung hyperinflation, accessory respiratory muscle use, muscle retraction, and pulsus paradoxus may be noted. Patients may attempt to sit upright or lean forward (tripod position) in an effort to ease the work of breathing. However, these signs may be absent despite severe airway obstruction. A silent chest indicates insufficient air movement or extensive mucous plugging and is an ominous sign. Cyanosis may appear only immediately before respiratory arrest and should not be relied on as an indication of the severity of the attack. The termination of an exacerbation is often marked by a cough productive of thick mucous plugs and bronchial casts (Curschmann spirals).
A subset of asthmatic patients experiences the sudden onset of severe symptoms. These individuals tend to respond rapidly to treatment but appear to be at significant risk for a fatal outcome.
Occasionally, an asthmatic patient may complain of intermittent dyspnea or cough on exertion. Patients with cough-equivalent asthma tend to have normal breath sounds on examination, even during an exacerbation, but reversible bronchospasm may be demonstrated on pulmonary function testing.
Complications of asthma include pneumothorax, pneumomediastinum, and subcutaneous emphysema. Pneumothoraces may require insertion of a chest tube for evacuation. Mild atelectasis may occur as a result of bronchial mucous plugging but rarely requires intervention other than conventional bronchodilator therapy. In addition, rib fractures and costochondral strain may occur as a result of excessive coughing. Cough syncope, a rare complication of asthma, is noted most frequently in moderately obese, middle-aged asthmatic men. Finally, dysrhythmias may occur as a result of hypoxia, especially in patients who have received oral adrenergic agents.
DIFFERENTIAL DIAGNOSIS
Wheezing, coughing, and dyspnea are present in many conditions. Common problems include pneumonia, bronchitis, croup, bronchiolitis, chronic obstructive lung disease, congestive heart failure, pulmonary embolism, allergic reactions, and upper airway obstruction from edema or a foreign body. Less common problems include cystic fibrosis, hypersensitivity pneumonitis, carcinoid syndrome, and exposure to odors, dust, and gas. A careful history and physical examination should help differentiate asthma from these conditions.
ED EVALUATION
The ability to perform a thorough history and physical examination before initiating treatment is often limited by the patient’s dyspnea. Aggressive therapy directed at relieving airway obstruction begins as soon as a diagnosis of asthma is established. Meanwhile, an attempt is made to obtain a history from family members or the patient. Important points to establish include the following (3,4).
• Duration and onset of the current attack
• Identification of precipitating causes
• Type and amount of medications (inhaler/nebulizer) used before arrival in the ED
• Response to prior therapy, including current or previous use of steroids
• Frequency of ED visits and hospitalizations
• Previous need for intubation or ventilation
• History of concurrent medications and allergies
• History of concurrent medical problems
• History of illicit drug use
Patients at high risk of asthma-related death include those with previous intubations; more than two hospitalizations or more than three ED visits in the past year; use of more than two canisters of short-acting β2-agonist (SABA) per month; difficulty perceiving airway obstruction or worsening asthma; low socioeconomic status or inner-city residence; illicit drug use; major psychosocial or psychiatric disease; age; and comorbidities such as cardiovascular or other chronic lung diseases.
Immediate attention is also directed to the patient’s appearance and work of breathing (i.e., use of accessory muscles), vital signs, chest, heart, mental status, and skin examination. The presence of a pulsus paradoxus (a drop of SBP of more than 12 mm Hg during inspiration) may be noted. However, judging the state of a patient’s ventilatory status and pulmonary function on clinical grounds alone can be misleading. The failure to recognize impending respiratory failure is a key contributor to patient death. The degree of tachycardia and tachypnea often do not correlate well with the degree of airway obstruction. Pulsus paradoxus may be absent in up to one-third of severe asthmatics (5). Furthermore, wheezing is absent if airflow is minimal. To complicate matters, all of the aforementioned clinical parameters can normalize with even minimal improvement in pulmonary function (6). Therefore, quantification of airway changes must be assessed by using either the forced expiratory volume in 1 second (FEV1) or the peak expiratory flow rate (PEFR). An initial FEV1 of <1 L (<30% predicted) or a PEFR of <100 L/min (<20% predicted) indicates very severe obstruction (3,4).
Hypoxemia is not common during most acute exacerbations. Pulse oximetry may be useful in assessing and following oxygenation. A saturation of <91% generally correlates with severe hypoxemia. Arterial blood gas (ABG) measurements are rarely needed in the management of acute asthma, unless the practitioner desires to know the pCO2 or pH. In general, hypercapnia, severe hypoxemia, or metabolic acidosis does not occur until the PEFR or FEV1 is <25% of predicted values (7), but young children and elderly patients commonly present exceptions to this rule. The degree of hypoxemia determined by ABG generally reflects the extent of ventilation–perfusion mismatch. A normal or increased PaCO2 indicates severe airway obstruction and impending ventilatory failure (7).
Expectorated sputum may appear purulent because of the presence of eosinophils, but this finding may not reflect infection. A wet preparation of sputum may contain Charcot-Leyden crystals (eosinophilic granules), Curschmann spirals (mucous casts), and bacteria.
Chest radiographs may demonstrate hyperinflation and atelectasis but are usually nondiagnostic. A chest radiograph is necessary only if pneumonia, pneumothorax, or pneumomediastinum is suspected or if the patient fails to respond to aggressive bronchodilator therapy.
Although electrocardiography occasionally reveals evidence of right ventricular strain, it is generally not helpful except to rule out concurrent cardiac problems. However, all older patients, especially those with cardiac disease, should be monitored during therapy.
Blood tests, including a complete blood count, are unlikely to help guide the acute management of an asthma attack. Asthma scores that incorporate subjective and objective criteria (pulse, respiratory rate, pulsus paradoxus, subjective dyspnea, accessory muscle use, wheezing, and PEFR) should not be relied on for predicting emergency treatment or determining disposition.
ED MANAGEMENT
ED interventions are guided by pulmonary function tests (FEV1, PEFR), vital signs, chest, heart and mental status examinations, and the patient’s subjective assessment of dyspnea. The goal of emergency treatment is to ensure adequate oxygenation and to relieve airflow obstruction.
Supplemental oxygen, inhaled β-adrenergic agonists, oral or intravenous corticosteroids, and, to a lesser extent, inhaled anticholinergic agents are the mainstays of asthma treatment in the ED. It is now thought that timely administration of glucocorticoids for serious exacerbations is the single most effective strategy for reducing ED visits and hospitalizations (8,9). The methylxanthines (e.g., theophylline) are not a component of emergency care (10). The benefits of ketamine remain largely anecdotal and have not been substantiated in controlled trials (11). The National Asthma Education and Prevention Program (NAEPP) Expert Panel (Fig. 76.1) (3,4) has established guidelines for the diagnosis and management of acute exacerbations of asthma. Acute pharmacologic therapy can be divided into three categories: β-adrenergic agonists, glucocorticoids, and anticholinergics. Chronic management is primarily composed of treatment with inhaled corticosteroids and long-acting inhaled β-agonists; however, these have no role in the acute setting. Despite effective therapies for chronic management, there is still frequently poor compliance, leading to poor control (1). Much work is being done to develop medications that further address the inflammatory component of asthma. This includes anti-IgE therapy and blockers of specific mediators (e.g., IL-5, 9, and 13) or receptors. These remain under investigation and their potential utility in the acute setting remains unclear (1).
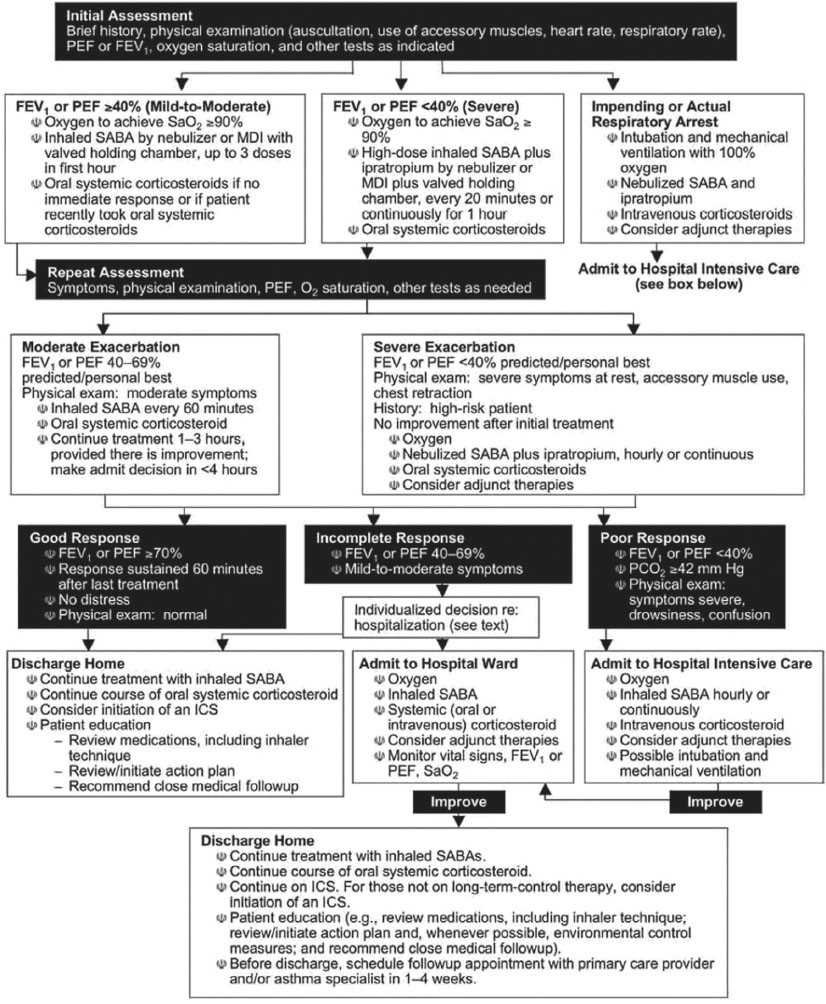
FIGURE 76.1 Management of asthma exacerbations: Emergency department and hospital-based care. Key: FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; MDI, metered-dose inhaler; PCO2, partial pressure carbon dioxide; PEF, peak expiratory flow; SABA, short-acting β2-agonist; SaO2, oxygen saturation. (Adapted from National Asthma Education and Prevention Program, Expert Panel Report 3: Guidelines of the Diagnosis and Management of Asthma (NIH Publication Number 08–5846). Baltimore, MD: Department of Health and Human Services, National Institutes of Health, 2007.)









