Ross Kerridge This chapter will describe: • developing a model of perioperative care • the implications of change for clinicians and managers • managing expectation and risk in the change process. The importance of appropriate preoperative and pre-anaesthetic patient assessment and preparation in order to assure patient safety has long been recognised, and has been confirmed by morbidity and mortality audits. In recent years, there has been an increased appreciation of the adverse effects of poor patient preparation on dimensions of quality other than safety, such as avoidable cancellations, process delays, inefficiency in the operating theatres, staff frustration, and patient dissatisfaction. Some of these effects may not be immediately apparent, but collectively produce a large negative impact on the quality of the health system. This is the inevitable result of a poorly designed and organised patient preparation system. Conceptually, it can be described as the ‘iceberg of poor patient preparation’ (Figure 17.1). Figure 17.1 The iceberg of poor patient preparation The traditional model of patient care for patients having surgery is based on the organisational structures developed in the hospitals of the late nineteenth century. In this model, it is presumed that the surgeon is in authoritative command of a small ‘firm’ that acts as a hierarchical team. This ‘firm’ was relatively autonomous with regard to the rest of the hospital – so that each surgeon or firm could have clinical practices that were unique to that firm. It was presumed unnecessary to involve other medical specialists in medical decision-making. The surgeon was regarded as omniscient, omni-competent, and omni-present – the traditional model assumes that the surgeon is always in control, and thus can and must be contacted (either directly or through a lower member of the hierarchy), whenever management decisions are to be made. The traditional model required the members of the surgical team to have a broad understanding of all matters to do with perioperative patient care, as the surgeon (or delegate) was empowered and expected to make decisions regarding any patient care matter. It also required the members of the surgical team to have a comprehensive knowledge of everything regarding the patient’s clinical care. These aspects of the traditional care model were major strengths, but were built on assumptions regarding the hospital workforce. It assumed that the patient was treated in one ward with a small stable number of nurses providing all the care on the ward. With regard to ‘junior’ members of the medical hierarchy, the traditional model implied a working style based on living in the hospital, and an expectation of being available and contactable at all times. This traditional model had both strengths and weaknesses. The major strengths of the traditional model included a clear line of command and control – it was clear to everyone in the system that one person was in charge of all decision-making. There was also clarity of process – the steps involved in an episode of surgical care could be described linearly, and there were very few points of ‘greyness’ where there were obviously conflicting requirements in planning patient care. The traditional model also existed in an environment that was different from that of today. Clinical information was less complex, as patients had fewer co-morbidities, inter-current therapies, and fewer results of investigations. The average length of stay was longer, and patients stayed on one ward for their entire hospital stay. Organisational process control (management of information) was substantially less. Information management was simpler due to the smaller number of clinicians and others involved in the care process. Finally, financial constraints and expectations of efficient process management were fewer. The results of poor patient preparation could be accommodated, by practices such as early admission, which are no longer accepted. Conceptually, the traditional surgical process was hospital-based; most steps in the process occurred in hospital (see Figure 17.2). After the initial decision that the patient needed an operation, the patient was admitted to hospital, and the care process commenced with nursing and medical admission, investigations, preparation, and the procedure itself. Postoperative care tended to be reactive, so that discharge planning started when the decision was made that the patient could be discharged. Reflecting this system, the portrayal of hospitalisation in popular culture has commonly featured the senior doctor announcing to the patient (and staff) ‘you can go home today’ as unexpected and welcome news. Figure 17.2 Traditional surgical process Figure 17.3 Traditional perioperative information flow The traditional process can also be viewed from an information management perspective (see Figure 17.3). The surgeon made the decision to operate at a particular time and informed the patient and the hospital. It was presumed that the patient’s health issues, personal preferences, the equipment and other requirements, and the organisational constraints of the hospital could all be managed as secondary to the original decision to operate. It can be seen that the traditional process was conceptually simple and linear, with few points of interactive or conflicting requirements. Information flowed in one direction, as planning was reactive. One possible exception arose with regard to anaesthetic management. In some countries (particularly in the British tradition), the anaesthetist was seen as professionally autonomous and independent of the surgeon, so that differing requirements or interpretations of information could result in a possible point of disagreement about clinical management. However, assessment for anaesthesia was the role of the procedural anaesthetist, who tended to become involved in care only shortly before surgery, and acted as a ‘journeyman’ or individualist practitioner. Decisionmaking options were thus often reduced to postponement or even cancellation – a tool of last resort used only when major patient safety issues were identified at the time of preoperative assessment by the anaesthetist. The traditional system is increasingly unable to deal with the complexities of modern patient care and the demands of the modern hospital workforce. These problems are becoming increasingly obvious, and despite its strengths, the traditional model is no longer sustainable. It is no longer possible for any single person to be omniscient and omni-competent with regard to all aspects of patient care. Multiple medical and non-medical specialists are involved in decision-making regarding patient care. Specialised knowledge is held by multiple semi-autonomous professions. The power hierarchy implied in the traditional model is neither appropriate nor acceptable in today’s multi-disciplinary healthcare teams. The individuals in the healthcare workforce are also changing. Nursing staff are better paid, have higher education levels, and have markedly different career and social expectations. The full-time (168 hours/week) commitment by medical staff that the traditional care model required is not compatible with current work- and life-style preferences. Allied health and ancillary staff are more commonly involved in patient care. The workforce is more specialised and fragmented, and more commonly working part-time. As a result, patient care is now delivered by multidisciplinary teams including a much larger number of staff, many of whom will have only transient contact with the patient. Apart from the changing health workforce, the organisational environment has also changed. Requirements for clinical information management are more critical as patients have more co-morbidities, and operations and surgical procedures are becoming more complex. This complexity is multiplied by patient care being geographically fragmented into specialised ward areas, particularly as length of stay is reduced. The rise of hospital management has made clinical process control more detailed, increasing organisation information requirements. There is less tolerance of process inefficiencies and other indicators of poor quality. Finally, the patient is no longer a passive ‘recipient’ of the outcomes of surgical or other healthcare processes, but is an active ‘partner in care’ whose preferences and choices must be included in planning and preparation for procedures. In order to deliver high-quality patient care for modern surgical and other procedures, with the modern health workforce, and in a changing hospital environment, there must be a fundamental redesign of the perioperative care process, and development of new roles for all health professionals in this process. Redesign of perioperative processes is occurring internationally with a wide variety of different changes, although common themes can be identified, which are discussed elsewhere (Chapter 18). Simultaneously, evidence is accumulating from disparate sources that can be used to guide decision-making as to how preoperative processes and systems should be designed. The remainder of this chapter will review the available evidence that can be used. Evidence is variously required for the purposes of clinical science (i.e. classical clinical research), to monitor and improve patient outcomes on a day-to-day basis (i.e. audit and quality improvement activities) and to provide a ‘hard’ basis to support a business case to be put to health service management to guide investment of resources in the preoperative service. Obviously, these different applications of ‘evidence’ have differing requirements, both in the importance given to the various outcomes measured, and the expectations of ‘scientific rigour’ in the data collection and analysis. Given that the change in perioperative care is an international phenomenon, what evidence is available? By the usual standards of evidence-based medicine, there is disappointingly little evidence that is generally (internationally) applicable to help make ‘hard’ decisions about the organisation and management of perioperative systems. This is a telling comment on the disparity between expectations of evidence in the ‘scientific’ world of clinical medicine and the standards expected in management. The most rigorous evidence deals with changing behaviours (and costs) in the area of preoperative testing. These various studies show clearly that an organised approach produces more appropriate care and saves costs (which is hardly surprising!), but doesn’t clarify which organisational model is best. There is limited evidence concerning other issues, such as reductions in cancellations on day of surgery, but most of the published work in this area is limited by being strongly affected by local conditions affecting organisation of services. Nevertheless, ‘evidence’ from disparate sources is available, and this provides some ‘hard’ data on which to base future action. It is useful to consider alternative strategies for analysing and presenting the data that is available. As noted above, management executives may be less concerned with ‘scientific’ data, and the subtleties of statistical analysis, than clinician/scientists. Furthermore, they may prefer to work from generalities to specifics by inductive reasoning, rather than the deductive processes of traditional scientific method. Conventional scientific analysis will start with ‘an open mind’, and gather data. Each piece of research evidence will be considered independently, and by deduction a general conclusion will be reached. Clinicians may tend to continue using this pattern when presenting data. Managers may prefer to use inductive methods, starting with a general theory or knowledge framework, and examining available data to draw conclusions using an inductive process. Selection of an appropriate framework is a key step in effectively presenting, analysing and using data inductively. This may be unfamiliar to traditional scientists, but is an effective strategy for using data in ‘grey’ areas such as clinical system redesign. A useful framework for assessing changes in clinical care systems is the patient-centred model of quality in healthcare, including seven elements of safety, access, effectiveness, efficiency, timeliness, acceptability and appropriateness.1 Figure 17.4 Seven elements of quality in healthcare Using the framework shown in Figure 17.4, evidence gathered to guide change in perioperative systems can be categorised, assessed and presented against these various elements of quality. The following brief review of existing evidence is not intended to be comprehensive, but rather to provide examples of the use of disparate sources of evidence presented within the suggested quality framework. ‘Inadequate preoperative assessment’ has been consistently identified as a contributing factor in adverse patient outcomes in multiple studies such as NCEPOD in the UK, the Australian Incident Monitorin Study (AIMS)2, and in in ongoing mortality reports in various jurisdictions3 and continues to be identified in hospital investigations. At the Cleveland Clinic, a shift to preoperative patient assessment and preparation by a centralised anaesthesiology-led service, rather than assessment by different surgical teams, led to a reduction in intraoperative cardiac events.4 Improved preoperative assessment may enable safer antibiotic choices. At the Mayo Clinic, patients at the preoperative evaluation clinic with a self-reported penicillin allergy were investigated preoperatively by consultation and skin testing.5 This enabled better identification of those who could be safely given beta-lactam antibiotics, reducing the use of vancomycin to only 16% of patients. The results of implementation of a re-engineered elective surgery service in an Australian tertiary referral hospital setting were studied.6 The hospital introduced pre-admission clinics and same day admission through a separate preoperative area. Previously, patients were admitted on the day before surgery through a surgical ward. There was no increase in adverse outcomes and an increase in patient satisfaction. Most interestingly, there was a major (almost 60%) reduction in indicators of surgical wound infection. This may be predominantly due to avoiding one night on a surgical ward preoperatively, although other factors may contribute. For the purposes of guiding change initiatives at an institutional or organisational level, these general findings can be supplemented by locally produced reports of adverse patient outcomes due to poor patient preparation identified within the organisation. A reduction in avoidable late cancellations and ‘no-shows’ of booked surgical patients is one of the most demonstrable benefits of improved preoperative preparation systems. This is probably best considered as addressing the ‘access’ element of the quality framework. Length of stay is also an ‘access’ issue, since it demonstrates that improved preparation systems can increase service provision within existing resources, and without adverse effects on other quality elements. This is one of the most powerful arguments for improved preoperative preparation. Early reports of changed preoperative systems showed reduced cancellations and length of stay.7,8 Some reports were equivocal.9 More recent reports from other jurisdictions have confirmed this effect. In the Netherlands, outpatient preoperative evaluation reduced cancellations from 2.0% to 0.9% and increased same day admissions.10 Reduction of avoidable cancellations by clinic-based preparation also improves theatre efficiency.11,12 Reduction of cancellations can be linked to cost savings.13 A recent survey of US Anaesthetists reported that cancellations and delays were substantially reduced in hospitals with preanaesthesia evaluation clinics, although problems persisted. Clinical information management deficiencies are a common cause of delays or cancellations.14 Apart from cancellations, hospital and operating theatre resources may be wasted by ‘no-show’ patients. A study in a US veterans’ administration hospital showed that patient non-appearance could be strongly predicted from non-compliance with clinic visits and other hospital procedures.15 By predicting such patients in advance, locally appropriate strategies can be implemented to avoid waste of resources and increase operating theatre utilisation. The characterisation of various causes of cancellations or ‘no-shows’ will vary widely depending on the jurisdiction and local factors, in particular the funding characteristics of the patient service provision. That said, cancellations due to whatever cause are a common cause of waste of resources or increased costs. The complexities of economic and business process modelling to demonstrate this comprehensively make interpretation difficult.16 As a result, the total impact of cancellations on theatre efficiency is commonly underestimated. Nevertheless, even ‘simple’ analysis, based on local audit, may produce powerful evidence of potential for improvement, and be used to emphasise the importance of improved patient preparation. Even in optimised systems, some late cancellations will be unavoidable, such as those due to patients developing unexpected acute illnesses. A ‘high-functioning’ perioperative service will include a ‘stand-by’ list of patients who have been identified in advance as appropriate, available and willing to be called at short notice to substitute for these late cancellations. With regard to preoperative assessment and preparation, what evidence is available that relates to the effectiveness of actions taken preoperatively? Some of the data refers to intermediate or surrogate outcomes, but there is increasing evidence that improved or redesigned systems for preoperative preparation result in increased effectiveness of actions at this stage in patient care. Improved preparation systems also increase the effectiveness of preoperative testing by increasing the likelihood that results are interpreted correctly and acted upon more rigorously.17 Importantly, medico-legal risk may be greater if a test is ordered and not acted upon than if it is not ordered at all. Outpatient preoperative assessment clinics are an effective means to reduce preoperative anxiety.18 Recent British experience supports the general effectiveness of consultant-led clinics for vascular surgery patients.19 A patient preparation system based on a multi-disciplinary clinic improved compliance with agreed guidelines giving beta-blockers perioperatively.20 Similarly, a system approach to clinical process redesign improved appropriate antibiotic selection and administration, resulting in reduced surgical site infections.21 Many enthusiastic health professionals have encouraged patients to quit smoking, but the effectiveness of such actions in the absence of a systematic approach is unclear. In the author’s hospital, the effectiveness of a multimodal preoperative smoking cessation programme including an interactive computer-based smoking assessment, brief advice, telephone follow-up, nicotine replacement therapy, and referral to follow-up telephone counselling has been evaluated. In a prospective randomised controlled trial, the programme resulted in 78% of smokers achieving preoperative cessation, with a three-month quit rate of 19%.22 The programme was acceptable to patients and staff and cost-effective. Apart from interest with regard to smoking cessation, this is a demonstration of the potential for improved patient care by utilisation of new technology, system redesign, and an organised approach to preoperative preparation. It is obvious that any treatment cannot be effective unless it is provided. The Institute for Healthcare Improvement has identified ten common patient care interventions for which there is evidence, but that are not routinely practised. An organised preoperative process may facilitate optimisation of patient healthcare with regard to these interventions. The efficiency of preoperative assessment and preparation systems has been most commonly evaluated by examining preoperative tests. This is a reflection of both a perception of widespread unnecessary preoperative testing, and the relative ease of studying the problem and effect of interventions.23 Commonly this is studied by audit of patient testing against defined ‘standards’ such as preoperative testing guidelines. Early reports of anaesthetist-led preoperative clinics showed reductions in unnecessary preoperative tests, with overall cost reductions.24 These results have been replicated by many others.25,26 The challenge of ensuring appropriate preoperative testing has been a major focus of discussion and a driver for system redesign, particularly in the USA. Preoperative testing is only a minor part of preoperative preparation and perioperative patient care, and the emphasis on this visible and easily measurable aspect may be to the detriment of overall system improvement. (In 1974 in my first year as a medical student, it was said that ‘Diagnosis is 90% history, 9% examination, and 1% tests’. This may have changed slightly but the message remains important.) Preoperative assessment requires clinicians to evaluate the patient by history and examination, not primarily by laboratory tests.27 In a widely noted British study that focused on the debate over different health disciplines in preoperative preparation clinics rather than process efficiency, nurses were shown to comply with testing guidelines more closely than ‘junior’ medical staff.28 In this study the nurses had been specifically trained to work in the preoperative clinic setting, whereas the surgical housemen were working in the traditional model of care, with minimal setting-specific training. The study was criticised as an examination of the effect of workforce substitution to increase process efficiency, without considering whether the process was perpetuating unnecessary rituals.29 The accuracy of trained nurses performing preoperative health assessment has also been studied.30 More recently, the use of a decision support system to assist trained nurses in making decisions with regard to preoperative investigations has been evaluated.31 Performance was compared to a reference standard based on the recommendations of multiple consultant anaesthetists after blinded assessment of case histories. The combination of the decision support system and nurses’ predictions achieved performance equivalent to consultant anaesthetists. Regardless of which health professionals are involved, these studies demonstrate that efficient and appropriate care is most readily achieved using a systemic approach with appropriately trained staff working in supervised teams. In a US tertiary hospital setting, implementation of a multi-disciplinary preoperative assessment and testing clinic resulted in fewer preoperative cardiology consults. The ordering of consults was not audited against a ‘gold standard’ but the results were interpreted as showing that unnecessary consults were avoided without adverse outcomes.32 Apart from preoperative testing, the efficiency of preoperative preparation may be evaluated by reduction in unnecessary clinic visits, process rework, and duplication of documentation. These indicators are more difficult to study, and there are few such reports in the formal scientific publications, although they are commonly mentioned in the ‘grey literature’. The use of quality management techniques to improve the efficiency of the preoperative assessment clinic itself has been advocated, particularly at the Cleveland Clinic.33 A recent report from the Netherlands discusses process management in preoperative clinic scheduling.34 The generalisability of the particular conclusions is limited by local factors, but these reports provide useful direction as to improvement of quality and efficiency in preoperative services. ‘Traditional’ surgical scheduling is based on clinical urgency. Existing scheduling systems can usually address clinical urgency appropriately. While clinical timeliness would generally be presumed to be of the highest priority, there may also be non-clinical factors that may determine optimal timing of surgery. Therefore ‘timeliness’ as an element of health service quality may also be determined by both organisational and patient factors. Optimising the scheduling of booked surgery may improve hospital processes by enabling proactive consideration of issues such as bed availability, planned utilisation of intensive care or high-dependency beds, improved operating theatre performance by aggregating or ordering cases appropriately, and assisting planning for equipment availability. From the patient’s point of view, timeliness may include scheduling of surgery to consider employment, social, transport or ‘simple’ personal preferences. This may include the planned day of operation, and timing on the day. A high-functioning perioperative service should identify both organisational and patients’ non-clinical preferences regarding scheduling of surgery. Some centres have described experience with this in the ‘grey literature’.35,36 There have been few high-quality studies of patient satisfaction with clinic-based preoperative assessment and preparation services.37,38 These few studies, and more general reports, have consistently reported high acceptability.39,40 Recent work from the Netherlands has provided detailed evaluation of patient preferences in the preoperative assessment clinic using a purpose-designed questionnaire.41 The variation in types of clinics, especially internationally, makes comparison difficult. Patients’ and health professionals’ perspectives on the important indicators of acceptability differ. Delays and waiting in clinics is the strongest source of dissatisfaction; patients value communication and choice about the clinic and hospital processes, being given information, and education about their planned procedure. Education aids such as printed booklets or videos can be used to increase patient satisfaction and the acceptability of the service.42 A recurrent finding in patient surveys and qualitative interviews is that patients do not like being asked the same question multiple times, and that inconsistent communication is a major source of concern and complaint. These patient preferences are facilitated by a well-organised preoperative assessment and preparation process. Most preadmission processes require some form of patient-completed health questionnaire. The development of electronic systems to improve the efficiency of this process has been reported by a number of groups. Patient acceptance of new technology has been seen as a constraint. A recent Canadian trial comparing paper and electronic questionnaires (PDA or touchscreen computer) found patients accepted electronic systems, and expressed a preference for computerised systems for future questionnaires.43 Anecdotal reports suggest that patients strongly prefer spending less time in hospital preoperatively. In the author’s hospital, discharge surveys of patient satisfaction after inpatient surgery have consistently found that over 90% of patients would prefer to arrive in hospital three hours or less before a planned operation, if it was medically acceptable. As discussed earlier, recent changes in preoperative processes represent a shift from passive and reactive hospital processes to more proactive systems. Centralisation of preoperative preparation provides a platform for oversight of planned surgery well before admission to hospital. This may enable the development of better processes to ensure that planned care is appropriate. Preoperative risk assessment can predict patients who are likely to have costly, prolonged or complex hospital treatment.44 Further, improved preoperative assessment can identify patients at high risk who may benefit from higher-intensity perioperative care, and may also identify patients who should not have surgery at all.45 Anecdotally, this is more likely to occur when performed early (as an outpatient) than immediately prior to proposed surgery, and established ‘high-functioning’ preoperative clinics report this latter function as a significant component of their work. Patients identified at high or even moderate risk may be appropriately encouraged to address end-of-life issues. Some experiences with programmes to facilitate advance care planning in the preoperative clinic setting have been reported.46 Improved preoperative assessment and preparation also provides an opportunity to explore appropriate new patient treatments. It is already known that there are a number of potential preoperative interventions to improve patient outcomes that are currently under-utilised. These interventions may have been viewed as not feasible or logistically difficult, or they may simply not be known about by the relevant clinicians. The development of improved, centralised systems for early preoperative assessment by specialists in this field makes it feasible to apply this knowledge. A number of ‘simple’ examples of utilisation of the preoperative period to improve or to provide ‘new’ treatments to improve patient outcomes have been reported from leading preoperative services in the USA.47 Other promising opportunities for improving patients’ health status and perioperative outcomes require further research and development. These are new specific interventions pertaining to the perioperative period. Examples include: • improved preoperative testing techniques (e.g. CPX testing) • better perioperative pharmacological therapy • preoperative exercise therapy • preoperative transfusion medicine • perioperative nutrition, diet and nutraceuticals • obesity management • novel uses of perioperative medication (e.g. anabolic steroids) • immunological interventions • preparation for convalescence. There is also a requirement for better research and integration of knowledge. For example, comprehensive risk/benefit assessment requires improved knowledge of the ‘natural history’ of the patient’s surgical disease (whether treated or not), and the effects of treatment on both survival and quality of life. This information then needs to be evaluated in partnership with the patient to enable genuinely patient-centred decision-making about appropriate patient care. This is not just ‘traditional’ preoperative preparation being performed with improved quality, but a new scope of clinical practice – the preoperative component of perioperative medicine. These improvements in patient outcome can be achieved with greatest quality and efficiency by using a coordinated multi-disciplinary team approach, tailored to the needs of individual patients. As noted earlier, redesign of preoperative processes is an international phenomenon. This implies a general acceptance that it must be ‘a good thing’. Clinicians with scientific training will seek evidence to advocate for and guide changes to the systems and processes that they are involved in. The evidence presented above, and from other sources, would appear to justify change. In particular, the economic justification would appear to be clear.48 Such evidence may not be sufficient. Hospitals, like all human organisations, are political environments. Changing an organisation is a political activity, and scientific evidence is only one part of effecting change. Two case studies in which the author was peripherally involved are presented as examples. 1. In Australia, most specialist doctors are remunerated by fee-for-service payments in accordance with a schedule of fees (the Medical Benefits Schedule) that are negotiated annually between the federal government and doctors’ industrial representatives. Since 1995, anaesthetists have been clinical leaders in driving the change in perioperative care systems in Australia, and have substantially changed their work practices as a result of these changes. In November 2006, the Departments of Treasury and Health agreed to changes in the fee for preoperative assessment from $35 to a complexity-graded fee to over $150 per patient, establishing parity with subspecialist physicians. Fees for postoperative consultations were also raised to parity. These changes happened because health bureaucrats recognised that clinical leadership had improved both patient care and the efficiency of the health system. The Treasury had been presented with a detailed economic analysis of the economic benefits of the perioperative system by the Australian Society of Anaesthetists. In addition to the evidence, however, there had been a sustained campaign of education, negotiation and lobbying of bureaucrats, parliamentarians and ministers for ten years prior to the decision. The evidence alone was not enough. 2. Hospitals in Australia are managed by State Departments of Health. In 2005, the Health Department in the largest state (New South Wales) convened a working party to define the best practice model for organisation of preoperative services. The Department anticipated some preconceived outcomes. The working party included a limited number of clinicians (nurses, anaesthetists, surgeons) as well as departmental officers. The clinicians on the working party brought their differing opinions, evidence and experience and laboriously negotiated with each other and the Departmental representatives. Additional clinicians were consulted. Finally, the group agreed on a general model of the preoperative process based on stated key principles, and recommended key performance indicators. This was published in a ‘Pre-Procedure Preparation Toolkit’, which was officially endorsed for general implementation by the Department.49 Although evidence was used substantially during the deliberations of the working party, the success of the exercise was substantially due to the prolonged commitment and negotiation – a political process – involving the members of the working party. In this case, the result was generally endorsed by all as ‘a good result’. It was, however, substantially different from outcomes that could have resulted otherwise. Again, the evidence alone was not enough. These examples are presented to illustrate the general point that advocacy of change is a complex challenge. The scientific milieu has produced heavy emphasis on the need for evidence, and a disciplined, rigorous approach to analysing and evaluating evidence. This is very appropriate, where it is possible, in the world of clinical science. Hence clinician scientists must develop skills in gathering, analysing and using evidence. By contrast, clinical process design and organisational management is far from an exact science. Clinicians wishing to manage perioperative systems must learn how to do so.50 Evidence must be used where possible, and this requires the skills of clinician scientists. Over and above this, however, other skills – and the commitment of time, effort, and persistence – are necessary to be effective in achieving change. 1. W. Runciman, A. Merry and M. Walton (2007). Safety and Ethics in Healthcare: Getting it Right. Aldershot: Ashgate Publishing. 2. M.T. Kluger, E.J. Tham, N.A. Coleman, W.B. Runciman and M.F.M. Bullock (2000). Inadequate pre-operative evaluation and preparation: a review of 197 reports from the Australian Incident Monitoring Study. Anesthesia 55: 1173–8. 3. Such as ‘Safety of Anaesthesia in Australia: A Review of Anaesthesia Related Mortality’. The Australian and New Zealand College of Anaesthetists, Melbourne 2002. 4. R.G. Borkowski, B.M Parker, B. Fitzsimmons and W.G. Maurer (2001). The incidence of cardiac related intraoperative quality indicators in ambulatory/same day surgery patients and inpatients after different preoperative evaluation processes. Anesthesiology 95: A32. 5. M. Park, P. Markus, D. Matesic and J.T. Li (2006). Safety and effectiveness of a preoperative allergy clinic in decreasing vancomycin use in patients with a history of penicillin allergy. Annals of Allergy, Asthma and Immunology 97: 681–7. 6. G.A. Caplan, A. Brown, P.J. Crowe, S-J. Yap and S. Noble (1998). Re-engineering the elective surgical service of a tertiary hospital: a historical controlled trial. Medical Journal of Australia 169: 247–51 7. S.P. Fischer (1996). Development and effectiveness of an anesthesia pre-operative evaluation clinic in a teaching hospital. Anesthesiology 85: 196–206. 8. R.K. Kerridge, A.L. Lee, E.M. Latchford, S.M. Beehan and K.M. Hillman (1995). The perioperative system: A new system for managing elective surgery patients. Anaesthesia and Intensive Care 23: 591–6. 9. J.B. Pollard and L. Olson (1999). Early outpatient preoperative anesthesia assessment: Does it help to reduce operating room cancellations? Anesthesia and Analgesia 89: 502–5. 10. W.A. Van Klei, K.G.M. Moons, C.L.G. Rutten, A. Schuurhuis, J.T.A. Knape, C.J. Kalkman and D.E. Grobbee (2002). The effect of outpatient preoperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay. Anesthesia and Analgesia 94: 644–9. 11. M.B. Ferschl, A. Tung, B.J. Sweitzer, D. Huo and D.B. Glick (2005). Preoperative visits reduce operating room cancellations and delays. Anesthesiology 103: 855–9. 12. D.J. Correll, A.M. Bader, M.W. Hull, C. Hsu, L.C. Tsen and D.L.Hepner. (2006). The value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency. Anesthesiology 105: 1254–9. 13. P. St. Jacques and M. Higgins (2004). Beyond cancellations: Decreased day of surgery delays from a dedicated preoperative clinic may provide cost savings. Journal of Clinical Anesthesia 16: 478–9. 14. N.F. Holt, D.G. Silverman, R. Prasad, J. Dziura and K.J. Ruskin (2007). Preanesthesia clinics, information management, and operating room delays: Results of a survey of practicing anesthesiologists. Anesthesia and Analgesia 104: 615–18. 15. M.D. Basson, T.W. Butler and H. Verma (2006). Predicting patient nonappearance for surgery as a scheduling strategy to optimize operating room utilization in veterans’ administration hospital. Anaesthesiology 104: 826–34. 16. C. McIntosh, F. Dexter and R.H. Epstein (2006). The impact of service-specific staffing, case scheduling, turnovers, and first-case starts on anesthesia group and operating room productivity: A tutorial using data from an Australian hospital. Anesthesia and Analgesia 103: 1499–516. 17. M.F. Roizen (2004). Preoperative evaluation. In R.D. Miller, ed. Anaesthesia (6th edn). Philadelphia: Churchill Livingstone: 927–97. 18. C.E. Klopfenstein, A. Forster and E. Van Gessel (2000). Anesthetic assessment in an outpatient consultation clinic reduces preoperative anxiety. Canadian Journal of Anesthesia 47: 511–15. 19. K.L. Cantlay, S. Baker, A. Parry and G. Danjoux (2006). The impact of a consultant anaesthetist led pre-operative assessment clinic on patients undergoing major vascular surgery. Anaesthesia 61: 234. 20. S. Armanious, D.T. Wong, E. Etchells, P. Higgins and F. Chung (2003). Successful implementation of perioperative beta-blockade utilizing a multi-disciplinary approach. Canadian Journal of Anaesthesia 50: 131–6. 21. G. Kanter, N.R. Connelly and J. Fitzgerald (2006). A system and process redesign to improve perioperative antibiotic administration. Anesthesia and Analgesia 103: 1517–21. 22. L. Wolfenden, J. Wiggers, J. Knight, E. Campbell, C. Rissel, R.K. Kerridge, A.D. Spigelman and K. Moore (2005). A programme for reducing smoking in preoperative surgical patients: Randomised controlled trial. Anaesthesia 60: 172–9. 23. J. Munro, A. Booth and J. Nicholl (1997). Routine preoperative testing: a systematic review of the evidence. Health Technology Assessment 1: 1–62. 24. M.A. Starsnic, D.M. Guarnieri and M.C. Norris (1997). Efficacy and financial benefit of an anaesthesiologist-directed university preadmission evaluation center. Journal of Clinical Anesthesia 9: 299–305. 25. L.M. Power and N.M. Thackray (1999). Reduction of preoperative investigations with the introduction of an anaesthetist-led preoperative clinic. Anaesthesia and Intensive Care 27: 481–8. 26. B.A. Finegan, S. Rashiq, F.A. McAlister and P. O’Connor (2005). Selective ordering of preoperative investigations by anesthesiologists reduces the number and costs of tests. Canadian Journal of Anaesthesia 52: 575–80. 27. M. Roizen (2000). More preoperative assessment by physicians and less by laboratory tests. New England Journal of Medicine 342: 204–5. 28. H. Kinley, C. Czoski-Murray, S. George, C. McCabe, J. Primrose, C. Reilly, R. Wood, P. Nicolson, C. Healy, S. Read, J. Norman, E. Janke, H. Alhameed, N. Fernandes and E. Thomas (2002). Effectiveness of appropriately trained nurses in preoperative assessment: randomised controlled equivalence/non-inferiority trial. British Medical Journal 325: 1323. 29. R.K. Kerridge (2003). Effectiveness of trained nurses in preoperative assessment. British Medical Journal 326: 600. 30. W.A. Van Klei, P.J. Hennis, J. Moen, C.J. Kalkman and K.G. Moons (2004). The accuracy of trained nurses in pre-operative health assessment: results of the OPEN study. Anaesthesia 59: 971–8. 31. B.V.S. Murthy, S.P. Lake and A.C. Fisher (2008). Evaluation of a decision support system to predict preoperative investigations. British Journal of Anaesthesia 100: 315–21. 32. L.C. Tsen, S. Segal, M. Pothier, L.H. Hartley and A. Bader (2002). The effect of alterations in a preoperative assessment clinic on reducing the number and improving the yield of cardiology consultations. Anesthesia and Analgesia 95: 1563–8. 33. W.G. Maurer, R.G. Borkowski and B.M. Parker (2004). Quality and resource utilisation in managing preoperative evaluation. Anesthesiology Clinics of North America 22: 155–75. 34. G.M. Edward, S.F. Das, S.G. Elkhuizen, P.J.M. Bakker, J.A.M. Hontelez, M.W. Hollman, B. Preckel and L.C. Lemaire (2008). Simulation to analyse planning difficulties at the preoperative assessment clinic. British Journal of Anaesthesia 100: 195–202. 35. NHS Modernisation Agency (2003). National Good Practice Guidance on Pre-operative Assessment for Inpatient Surgery. Operating and Pre-operative Assessment Programme. London: Department of Health. 36. A. Bassett (2007). Two steps forward, One step back. Todmorden: Perigon Health Care Ltd. 37. D.L. Hepner, A.M. Bader, S. Hurwitz, M. Gustafson and L.C. Tsen (2004). Patient satisfaction with preoperative assessment in a preoperative assessment testing clinic. Anesthesia and Analgesia 98: 1099–105. 38. G.M. Edward, J.C.J.M. de Haes, F.J. Oort, L.C. Lemaire, M.W. Hollman and B. Preckel (2008). Setting priorities for improving the preoperative assessment clinic: the patients’ and professionals’ perpective. British Journal of Anaesthesia 100: 322–6. 39. B.V.S. Murthy (2006). Improving the patient’s journey. The role of the pre-operative assessment team. Bulletin of the Royal College of Anaesthetists 37: 1885–7. 40. E.P. Wittkugel and A.M. Varughese (2006). Pediatric preoperative evaluation: A new paradigm. International Anesthesiology Clinics 44: 141–58. 41. G.M. Edward, L.C. Lemaire, B. Preckel, F.J. Oort, M.J.L. Bucx, M.W. Hollmann and J.C.J.M. de Haes (2007). Patient experience with the Preoperative Assessment Clinic (PEPAC): validation of an instrument to measure patient experiences. British Journal of Anaesthesia 99: 666–72. 42. A. Cheung, B.A. Finegan, C. Torok-Both, N. Donnelly-Warner and J. Lucig (2007). A patient information booklet about anaesthesiology improves preoperative patient education. Canadian Journal of Anaesthesia 54: 355–60. 43. E.G. VanDenKerkhof, D.H. Goldstein, W.C. Blaine and M.J. Rimmer (2005). A comparison of paper with electronic patient-completed questionnaires in a preoperative clinic. Anesthesia and Analgesia 101: 1075–80. 44. D.L. Davenport, W.G. Henderson, S.F. Khuri and R.M. Mentzer (2005). Preoperative risk factors and surgical complexity are more predictive of costs than postoperative complications: a case study using the national surgical quality improvement program (NSQIP) database. Annals of Surgery 245: 463–71. 45. S.J. Davies and R.J.T. Wilson (2004). Preoperative optimization of the high-risk surgical patient. British Journal of Anaesthesia 93: 121–8. 46. D.A. Grimaldo, J.P. Wiener-Kronish, T. Jurson, T.E. Shaughnessy, J.R. Curtis and L.L. Liu (2001). A randomized, controlled trial of advance care planning discussions during preoperative evaluations. Anesthesiology 95: 43–50. 47. A.K. Jaffer and F.A. Michota (eds) (2007). Proceedings of the 3rd Annual Perioperative Medicine Summit. Cleveland Clinic Journal of Medicine 74: E-Supplement 1 (and preceding years). 48. G.L. Gibby (2002). How preoperative assessment programs can be justifued financially to hospital administrators. International Anesthesiology Clinics 40: 17–30. 49. Available free from the NSW Department of Health. Use of a general search engine (e.g. Google) and the term ‘Pre-Procedure Preparation Toolkit’ is suggested. 50. A.P. Harris and W.G. Zitzmann (1998). Operating Room Management: Structure, Strategies and Economics. St. Louis: Mosby Year-Book, Inc.
Chapter 17 Changing the preoperative process – a review of the evidence
SUMMARY
INTRODUCTION
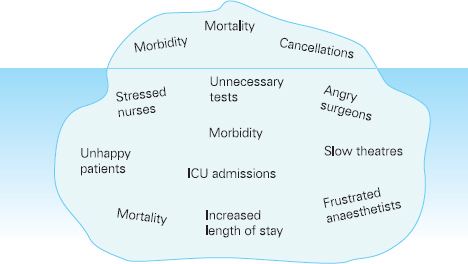
The traditional preoperative process
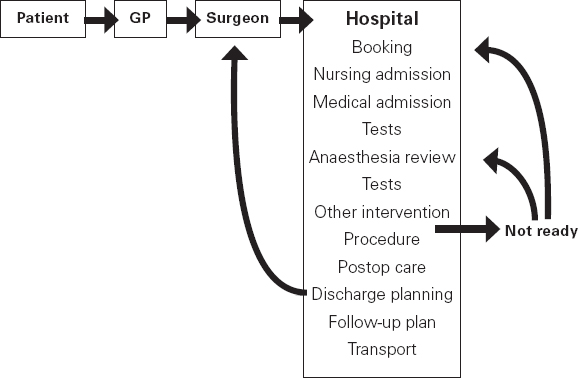
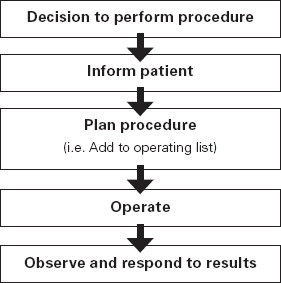
The traditional process is failing
Using evidence for managerial decision-making
Presenting the available evidence in a quality framework
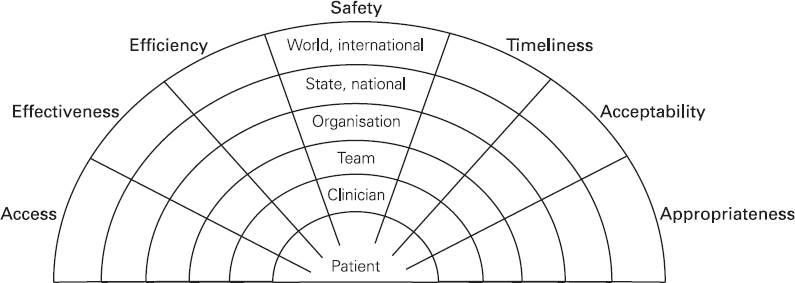
Safety – Does it do no harm, and/or reduce harm being done?
Access – Is the patient able to access the clinical service provided?
Effectiveness – Does the treatment provided achieve the intended result?
Efficiency – Are unnecessary or ineffective actions avoided?
Timeliness – Is the service provided at the ‘right’ time?
Acceptable – Is the service acceptable to patients?
Appropriate – Is the choice of treatment appropriate for the patient?
When evidence is not enough
REFERENCES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





