Chapter 65 Central Nervous System Infections Presenting to the Pediatric Intensive Care Unit
Understanding infections of the CNS entails more than a comprehension of the appropriate antimicrobial agents and laboratory tests. Recently it has become clear that CNS infections in childhood may have long-term consequences. Earlier studies focused on risk exposures of congenital infections of the CNS acquired during the fetal period. However, the human brain continues to develop well into early adulthood, and infections of the CNS during infancy and childhood may increase the risk of neuropsychiatric problems later in life. A study from South America of persons who had “epidemic meningitis” during childhood (mainly of bacterial origin) demonstrated a fivefold increase in the prevalence of psychotic disorders.1 Additionally, in a recent Swedish study of over 1 million pediatric patients, serious viral CNS infections during childhood, specifically mumps virus and cytomegalovirus (CMV), appeared to be associated with the later development of schizophrenia and other psychoses.2 The exact pathophysiology of these long-term insults is currently the focus of clinical interest. Use of new immunohistochemical staining, such as amyloid precursor protein (β-APP), along with biomarkers of axonal injury such as c-tau and α-II spectrin, may offer insight into the axonal and neuronal injury that occur both with and following CNS infections.3,4
Bacterial Meningitis
Epidemiology
The use of conjugate vaccines against Haemophilus influenzae type b (Hib) and Streptococcus pneumoniae have significantly decreased the incidence of Hib as a meningeal pathogen and S. pneumoniae as a cause of invasive disease.5 Even as nonvaccine serotypes have emerged as “newly recognized” pathogens, S. pneumoniae remains an important adversary.6,7 The isolation of nonvaccine serotypes such as 19A, 22F, and 35B has increased since the introduction of the seven-valent vaccine. It is of great therapeutic concern that approximately 28% of these invasive isolates are resistant to penicillin and 11.8% are resistant to cefotaxime. Neisseria meningitidis is the second most common cause of sepsis and meningitis beyond the neonatal period. In a recent study of children with bacterial meningitis, one third of cases were by S. pneumoniae, while N. meningitidis was responsible for 28% of cases. Sixty-two percent of typed pneumococcal isolates were caused nonvaccine serotypes.8
In young infants in the first few months of life, Escherichia coli has become the main cause of sepsis and meningitis and is responsible for 22% of cases. Because of the use of intrapartum prophylaxis, Streptococcus agalactiae (group B Streptococcus [GBS]) is being observed less frequently.9 Other organisms such as Klebsiella pneumoniae, Enterobacter cloacae, Cronobacter spp. (formerly known as Enterobacter sakazakii),10 and Citrobacter koseri (formerly known as C. diversus) have been reported as causes of meningitis in young infants.
Children with asplenia, humoral immunodeficiencies, cochlear implants, human immunodeficiency virus (HIV), and cerebrospinal fluid (CSF) leakage resulting from trauma are at high risk of pneumococcal meningitis. Asplenic children and those with complement deficiencies are at risk for meningococcal meningitis. On rare occasions, Streptococcus pyogenes has been responsible for meningitis, with concurrent otitis media being a major risk factor.11 S. pyogenes was responsible for close to 2% of cases. Less frequent meningeal pathogens in young infants are Salmonella species and Capnocytophaga canimorsus, an organism associated with exposure of the mucous membranes to dog saliva.12
Clinical Manifestations
The classic features of bacterial meningitis such as fever, nuchal rigidity, and altered mental status may not always be observed in young infants and children. Seizures, irritability, decreased appetite, vomiting, poor perfusion, hypotension, coma, and respiratory distress may indicate a serious bacterial infection. Paleness and skin mottling may be observed in severely ill infants. Nuchal rigidity and bulging fontanelle are usually late manifestations of meningitis. Kernig and Brudzinski signs are frequently unrecognized or are absent. The presence of petechiae and purpura may suggest meningococcus as the causative agent. However, similar findings have been observed in children with S. pneumoniae and H. influenzae (type a) sepsis.13 Anisocoria, poorly reactive pupils, bulging fontanelle, diplopia, papilledema, and uncontrollable vomiting are signs of increased intracranial pressure (ICP) (see also Chapter 59, Intracranial Hypertension and Brain Monitoring).
Diagnosis
Lumbar puncture should be postponed in children with an ongoing coagulopathy, elevated ICP, or cardiorespiratory difficulty, and in patients with suspected mass-occupying lesions as demonstrated by focal neurologic signs. However, it is critical to remember that antimicrobial therapy and other supportive measures should not be delayed while waiting for results of CSF analysis. Routine end-of-treatment lumbar punctures are no longer recommended.14 Patients with multidrug resistant S. pneumoniae or Gram-negative bacilli meningitis should have a repeat lumbar puncture at 48 hours after initiation of therapy to document sterilization.
Although a computed tomography (CT) scan is not required in all children with suspected meningitis, it is indicated prior to lumbar puncture in patients with focal neurologic findings and signs of ICP. It is important to remember that even when CT scans are normal, herniation may still occur.15 Similar findings have been confirmed in adults with meningitis.16
Gram stains have been reported to demonstrate organisms in 50% to 75% of specimens. The impression of most clinicians is that it is not that high. The clinician is cautioned not to make significant changes in antimicrobial therapy based solely on a Gram stain result. CSF cultures remain the ultimate gold standard.
Routine use of rapid bacterial antigen detection assays have been shown to be of limited clinical relevance because on most occasions therapy typically is not altered based on the result.17 However, in a multisite study from Asia and Africa, an immunochromatographic test for pneumococcal antigen was found to have high sensitivity and specificity. This assay may be useful in previously treated children with a negative CSF Gram stain and culture.18
Real-time fluorescent quantitative polymerase chain reaction (PCR) amplification of the bacterial 16S ribosomal ribonucleic acid (RNA) gene may become a useful clinical tool for the rapid diagnosis of bacterial meningitis. It would be particularly beneficial in patients who have taken antibiotics prior to undergoing a lumbar puncture. Compared with bacterial culture controls, sensitivity was 100% in one study; in another study it was 86% overall.19,20 This assay also may be useful for the prompt identification of S. pneumoniae and N. meningitidis as the etiologic agents.21 In a clinical setting, a real-time PCR for N. meningitidis was found to have a high sensitivity, specificity, and predictive values.22
Serum procalcitonin assays have been used to differentiate between bacterial and aseptic meningitis. In a small study, procalcitonin determination had 99% sensitivity and 83% specificity.23 A larger prospective study will be needed to assess the ultimate clinical role for this assay.
Treatment
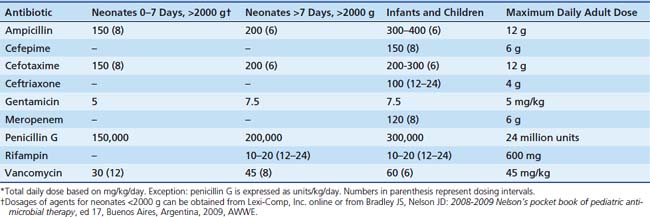
The Infectious Disease Society of America has published practice guidelines for the management of bacterial meningitis. All critical care clinicians should be familiar with their recommendations.24 Tables 65-1, 65-2, and 65-3 provide a listing of most frequent meningeal pathogens and recommended antimicrobial regimens along with dosage information.
Table 65–1 Initial Empiric Antimicrobial Therapy for Bacterial Meningitis Based on Presumptive Pathogen(s)
| Age and/or Predisposing Condition | Pathogen | Antimicrobial Therapy |
|---|---|---|
| <1 mo | Escherichia coli, Streptococcus agalactiae, Klebsiella spp., Listeria monocytogenes | Ampicillin + cefotaxime ± aminoglycoside |
| 1–2 mo | Streptococcus pneumoniae, Neisseria meningitidis, S. agalactiae, Haemophilus influenzae, E. coli | Vancomycin + third-generation cephalosporin∗ |
| 2 mo–5 y | S. pneumoniae, N. meningitidis, H. influenzae | Vancomycin + third-generation cephalosporin∗ |
| >5 y | S. pneumoniae, N. meningitidis | Vancomycin + third-generation cephalosporin∗ |
| Humoral immunodeficiency, human immunodeficiency virus, asplenia | S. pneumoniae, N. meningitidis, H. influenzae, Salmonella spp. | Vancomycin + third-generation cephalosporin∗ |
| Complement deficiencies | N. meningitidis, S. pneumoniae | Vancomycin + third-generation cephalosporin∗ |
| Basilar skull fractures | S. pneumoniae, N. meningitidis, Streptococcus pyogenes | Vancomycin + third-generation cephalosporin∗ |
| Cerebrospinal fluid, shunt related | Coagulase-negative staphylococci, Staphylococcus aureus (methicillin-susceptible, methicillin-resistant), aerobic gram-negative bacilli (including Pseudomonas aeruginosa), Propionibacterium acnes | Vancomycin + ceftazidime or vancomycin + cefepime or vancomycin + meropenem† |
| After neurosurgery | Coagulase-negative staphylococci, S. aureus (methicillin-susceptible, methicillin-resistant), aerobic gram-negative bacilli (including P. aeruginosa), P. acnes | Vancomycin + ceftazidime or vancomycin + cefepime or vancomycin + meropenem† |
| Cochlear implants | S. pneumoniae | Vancomycin + third-generation cephalosporin∗ |
† The choice of anti–gram-negative bacillary agent should be based on the institution’s susceptibility patterns.
Table 65–2 Antimicrobial Therapy for Specific Meningeal Pathogens
| Pathogen | Antimicrobial Therapy |
|---|---|
| Streptococcus agalactiae | Penicillin G ± gentamicin or ampicillin ± gentamicin |
| Hemophilus influenzae, β-lactamase–negative | Ampicillin |
| H. influenzae, β-lactamase–positive | Third-generation cephalosporin∗ |
| Streptococcus pneumoniae, penicillin-susceptible | Penicillin G or ampicillin† |
| S. pneumoniae, penicillin-resistant, cephalosporin-susceptible | Third-generation cephalosporin∗ |
| S. pneumoniae, drug resistant (multiply resistant) | Vancomycin + rifampin |
| Neisseria meningitidis | Third-generation cephalosporin∗ |
| N. meningitidis, penicillin-susceptible | Penicillin G or ampicillin† |
| Escherichia coli, other aerobic enteric gram-negative bacilli (not including Pseudomonas aeruginosa) | Cefotaxime or ceftriaxone (if >1 mo of age) + aminoglycosideठ|
| P. aeruginosa | Meropenem + aminoglycoside or cefepime + aminoglycoside or ceftazidime + aminoglycoside§ |
| Coagulase-negative staphylococci, methicillin-resistant Staphylococcus aureus | Vancomycin ± rifampin |
| Methicillin-susceptible S. aureus | Nafcillin |
† Some clinicians prefer ampicillin because of less frequent dosing.
‡ The choice of aminoglycoside will depend on the institution’s susceptibility patterns.
§ The choice of antipseudomonal regimen is influenced by the institution’s susceptibility patterns.
In the neonate with meningitis, empiric antimicrobial agents used initially should consist of ampicillin, an aminoglycoside, and cefotaxime. In infants older than 1 month, vancomycin and a third-generation cephalosporin (cefotaxime or ceftriaxone) is recommended. Alterations to these regimens may be influenced by Gram stain, culture, and drug susceptibility results. Additional empiric agents could be added according to epidemiologic exposures, underlying conditions, and the presence of resistant organisms in the community or hospital.
Supportive Care
In the patient who is dehydrated or in shock, administration of intravenous fluids is critical for the maintenance of normal blood pressure and proper perfusion; in addition, it potentially reduces the risk of central venous or sagittal sinus thrombosis. Some patients may require vasoactive-inotropic agents such as dopamine and dobutamine. Routinely restricting fluids in patients with CNS infection as a way to prevent inappropriate antidiuretic hormone secretion should be limited. Patients may have elevated ICP as a consequence of CNS inflammation or obstruction by purulent debris of their lateral, third, or fourth ventricles. Treatment of elevated ICP is discussed in Chapter 59, Intracranial Hypertension and Brain Monitoring. Anticonvulsant therapy is indicated in patients with seizures. Generalized seizures are present in ~20% to 30% of patients in the first 3 to 4 days of illness. The presence of focal seizures and an onset past the third day of illness are associated with long-term neurologic sequelae.
Adjunctive Therapy
Two agents, glycerol and dexamethasone, have been evaluated as candidates for possible adjuvant therapy in persons with meningitis. In various studies, orally administered glycerol had been shown to reduce neurologic sequelae in children with bacterial meningitis. It has been postulated that through an increase in serum osmolality, enhanced movement of water back into plasma would result in a reduction of CSF volume and an increase in cerebral blood flow.25,26 However, in a recently published multicenter trial, it was found that glycerol did not prevent hearing loss, which is a frequent neurologic sequelae.27
Corticosteroids such as dexamethasone (DXM) have a discernible effect on inflammatory markers in the CSF in persons with bacterial meningitis.28 Use of DXM in children remains a topic of controversy among clinicians. In adults with pneumococcal meningitis, the early administration of DXM (15 to 20 minutes before or with the first dose of an antibiotic) was associated with a reduction in mortality and unfavorable outcomes.29
In early studies in children with Hib meningitis, children treated with DXM had a lower incidence of moderate-to-severe hearing deficits when compared with placebo.30 However, one of the antimicrobial agents used in the study, cefuroxime, was later shown to be associated with increased neurologic sequelae. This single factor may have influenced the results for the placebo group. In a study by Peltola and Roine, DXM did not prevent neurologic sequelae in children with Hib meningitis; however, glycerol performed in a favorable manner.31 On the other hand, in a recently published multicenter study, neither agent was found to prevent hearing loss.27
In children with pneumococcal meningitis, DXM has not demonstrated the same magnitude of benefit as in adults. In one study, DXM use was associated with an increase in moderate or severe hearing loss and other neurologic deficits.32 As a consequence, some clinicians may elect not to administer DXM. However, many experts would agree to administer DXM if Hib meningitis was suspected (e.g., in an unimmunized child, in a child living in an endemic region with known Hib transmission, or in a child whose Gram stain shows pleomorphic gram-negative bacilli).33
There is no evidence that corticosteroids are beneficial in patients with viral or meningococcal or neonatal meningitis.34,35 In contrast, patients with tuberculous meningitis who are treated with steroids have an improved survival rate compared with those who are not treated with steroids. However, the severity of disability commonly observed in those with tuberculous meningitis is not altered.36
Prevention
Persons such as household members, caregivers, and day care center playmates who have significant prolonged and close exposures to children with N. meningitidis meningitis should receive antimicrobial prophylaxis with either ceftriaxone, ciprofloxacin, or rifampin.37 Recently ciprofloxacin resistance was reported in Minnesota and North Dakota. Clinicians should be familiar with resistance rates in their communities.38 Young children exposed to a person with Hib meningitis also may require prophylaxis with rifampin to eradicate potential nasopharyngeal colonization and prevent secondary infections.39
Outcomes
No single laboratory factor is entirely predictive of outcome in children with meningitis. In pneumococcal meningitis, antimicrobial resistance was not associated with death, ICU admission, need for mechanical ventilation, focal neurologic deficits, seizures, secondary fevers, or duration of hospital stay.40 However, the presence of shock, hyponatremia, or coma upon admission to the ICU was associated with a higher use of invasive medical devices and higher mortality.41,42 The presence of low leukocyte blood cell and platelet counts also was associated with increased mortality.43 In another study, no child with a leukocyte blood cell count greater than 16,000/μL died.44
Subdural Empyema
Subdural collections complicating meningitis are common in young infants, and they occur in 40% of infants with proven pyogenic meningitis.45 However, these fluid collections are typically sterile and cause no long-term sequelae. Subdural empyemas (SDE) are serious CNS infections defined as purulent fluid collections outside the brain parenchyma but contained under the dura. They usually are encapsulated and often loculated. Historically, otorhinolaryngeal infections were an important predisposing factor to SDE in older children. However, a recent study demonstrated that only 10% of episodes were related to otorhinolaryngeal infections. The decrease in the incidence of SDE after sinus infections is presumed to be from increased antibiotic use for this disease process. Meningitis, head trauma, or neurosurgeries are now more common predisposing conditions for SDE.46
In adolescents and adults, subdural empyemas usually result from infection of the paranasal sinuses, middle ear, and face or, less frequently, from a penetrating skull fracture. In young infants, SDE is rare and is usually a complication of purulent meningitis wherein the infection extends through the arachnoid and into the subdural space.47 The sequelae of SDE may be more severe in young infants than in any other age group.48 The clinical symptoms can mimic mild meningitis, followed several days later by rapidly progressive drowsiness, neurologic deficits, and seizures. Prolonged fever (90%), seizures (70%), and focal neurologic signs (60%) are the most common clinical signs noted in the pediatric population.46
In children, SDE is commonly secondary to Hib or S. pneumoniae meningitis. SDE also occurs with Salmonella, N. meningitidis, E. coli, and neurotuberculosis. The most common pathogen in infants younger than 4 months of age is GBS.46
The diagnosis is often made with radiologic assistance and evaluation of the subdural fluid collection. Distinguishing an SDE from a sterile reactive subdural effusion (RSE). is often difficult.49 The differentiation of SDE from sterile RSE may not be possible if contrast enhancement of the inner membrane is not seen at CT. Magnetic resonance imaging (MRI) is superior to CT in the demonstration of both the extra-axial fluid and the enhancement of the inner membrane with a paramagnetic contrast medium. Still, there are no MRI characteristics that are specific for SDE compared with a nonpurulent RSE. Analysis of the subdural fluid often is required to establish the diagnosis of SDE. Serial cranial ultrasounds may be the modality of choice for the evaluation of response to medical management.48
The keys to an optimal outcome in SDE are early, accurate diagnosis, timely intervention, and appropriate antibiotic therapy. The reported mortality rate of SDE is approximately 10%.46 Age, level of consciousness, timing and aggressiveness of treatment, and the rapidity of disease progression all influence outcome.
Meningoencephalitis
While the term meningitis implies that the meninges are primarily involved, encephalitis indicates brain parenchymal involvement. The presence or absence of normal brain function is important in distinguishing between aseptic meningitis and encephalitis. Patients with meningitis have fever, meningeal symptoms, and CSF pleocytosis but no evidence of bacterial or fungal infection and no associated neurologic dysfunction.50 Abnormal neurologic function, such as focal neurologic signs, cranial nerve involvement, motor deficits, sensory deficits, or speech difficulties, implies brain parenchymal involvement and encephalitis. Both meningitis and encephalitis may result in increased ICP, and patients may present with coma as a result of increased ICP or partial or complete brainstem herniation. Patients presenting with seizures may have meningitis or encephalitis. Making the distinction between meningitis and encephalitis is often difficult, and patients may present with both a parenchymal and meningeal process. Therefore, some clinicians prefer the term meningoencephalitis to recognize the inherent overlap between the two clinical entities.
Epidemiology
A survey of hospital discharge records from 1988 to 1997 showed approximately 19,000 hospitalizations per year (7.3 hospitalizations per 100,000 population), 230,000 hospital days, and 1400 deaths annually due to viral encephalitis in the United States.51 Children younger than 1 year had the highest risk for hospitalization as a result of encephalitis. Between the years 1989 to 1998, approximately 1100 deaths were attributed to encephalitis in children younger than 19 years in the United States. The highest rate of death occurred in children younger than 1 year (9.3 per 100,000 population).52 Nonviral causes of encephalitis are extremely rare.
Although the causative agent in many cases of encephalitis can be difficult to isolate, most cases are attributed to enteroviruses, herpesviruses, and arboviruses. Table 65-4 lists some of the common pathogens associated with meningoencephalitis. The enteroviruses and arboviruses in particular display seasonality in infection, being seen most frequently in the summer and autumn months. Herpesviruses, CMV, varicella-zoster virus (VZV), and Epstein-Barr virus (EBV) often show a predilection for the winter and spring. Herpes simplex virus (HSV), on the other hand, does not demonstrate any seasonality. With this epidemiology in mind, the evaluation of children with suspected viral meningoencephalitis is guided by many factors, including age, geographic location, season of the year, vaccination status, chronic conditions or immunosuppression, history of tick or mosquito exposure, and travel history.
Table 65–4 Causes of Meningoencephalitis and Available Laboratory Testing and Treatment
| Virus | Available Laboratory Testing | Treatment |
|---|---|---|
| COMMON CAUSES | ||
| Enterovirus | No specific therapy | |
| Poliovirus (three serotypes) | Cell culture; isolates sent to CDC via state laboratories | |
| Nonpoliovirus | CSF PCR | |
| Echovirus (28 serotypes) | Cell culture, CSF PCR | |
| Coxsackievirus A (23 serotypes) | CSF PCR | |
| Coxsackievirus B (six serotypes) | Cell culture, PCR | |
| Parechovirus (six serotypes) | None | |
| Herpesvirus | ||
| Herpes simplex virus-1, -2 | CSF PCR | Acyclovir, foscarnet (if resistant to acyclovir in immunocompromised patients) |
| Cytomegalovirus | CSF PCR | Ganciclovir, Foscarnet |
| Ebstein-Barr virus | CSF PCR | |
| Varicella zoster | CSF PCR | Acyclovir |
| Human herpesvirus-6 | CSF PCR | |
| Arbovirus | Viral isolation, serology done by state and research laboratories, detection of virus-specific IgM in CSF + Ab in serum specimen; CSF PCR in reference laboratories | |
| Flavivirus | ||
| St. Louis encephalitis | ||
| West Nile virus | ||
| Togavirus | ||
| Eastern equine encephalitis virus | ||
| Western equine encephalitis virus | ||
| Bunyavirus | ||
| California encephalitis virus | ||
| La Crosse virus | ||
| Jamestown virus | ||
| Snowshoe virus | ||
| LESS COMMON CAUSES | ||
| Mumps | Cell culture, mumps-specific IgM Ab, PCR | |
| Adenovirus | CSF PCR, cell culture | None |
| Respiratory syncytial virus | Viral isolation from nasopharyngeal secretion in cell culture, PCR | |
| Influenza A and B | Viral culture, PCR | Oseltamivir, zanamivir, amantadine or rimantadine if susceptible |
| HIV | HIV-1 nucleic acid detection by PCR | Multiple drug regimens |
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|