158 Cardiovascular and Endocrinologic Changes Associated with Pregnancy
 Cardiovascular Changes in Pregnancy
Cardiovascular Changes in Pregnancy
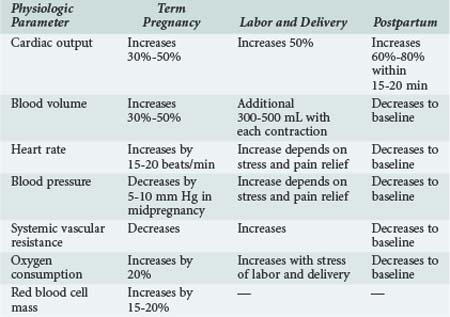
Cardiovascular and blood volume changes are among some of the more dramatic changes that occur in pregnancy (Table 158-1). These changes are primarily adaptive mechanisms, allowing the pregnant woman to accommodate her additional metabolic needs as well as those of the fetus during gestation and immediately after delivery. Cardiac output is significantly increased during pregnancy by as much as 50% compared with nonpregnant values. Cardiac output is further increased in twin pregnancies and multiple gestations.1,2 The dramatic rise in cardiac output is seen as early as the first 6 to 8 weeks of pregnancy. After the 10th week, cardiac output is increased by 1 to 1.5 L/min and reaches a maximum value by approximately the 20th to 24th week of gestation. The early increase in cardiac output is primarily due to a significant increase in stroke volume. However, stroke volume decreases as the pregnancy advances because of aortocaval compression by the uterus and the pressure of the fetal presenting part on the common iliac vein. Caval compression occurs because the large, gravid uterus rests on the vena cava, effectively decreasing venous return to the heart and therefore decreasing ventricular preload. In the latter half of pregnancy, a progressive increase in the maternal heart rate by 15 to 20 beats/min is primarily responsible for maintaining the elevated cardiac output. The additional increase in cardiac output before labor and delivery is caused by a further increase in heart rate. Resting cardiac output either is maintained or decreases slightly as term approaches.3
Influence of Body Position
Venous return is further compromised with changes in body position, particularly if the pregnant patient is supine. As a result, cardiac output can be diminished by as much as 25% to 30%. The effects of changes in body position are most obvious in the latter half of pregnancy when the fetal size and gravid uterus can effectively tamponade the vena cava. This phenomenon is exaggerated in women with poorly developed venous collaterals. With compression of the vena cava in the supine position, these women exhibit signs of severe hypoperfusion (hypotension and bradycardia), a phenomenon described as the supine hypotensive syndrome of pregnancy. Symptoms quickly resolve after the patient is repositioned to the left lateral recumbent position.4 Cardiac output can decrease by 30% to 40% in patients with this syndrome. This vasovagal phenomenon underscores the influence of maternal body position on the hemodynamic alterations occurring in pregnancy.
Hemodynamic changes associated with a decrease in preload and, subsequently, a reduced cardiac output are less pronounced when the gravid uterus is minimally compressing the vena cava. This is optimally achieved by maintaining the pregnant woman with more than 20 weeks gestation in the full left lateral position whenever she is recumbent. Alternatives to this position, less optimal than the left lateral position but preferable to the supine position, are a left lateral tilt to 15 degrees or manual displacement of the gravid uterus. The latter maneuver of left uterine displacement can be performed by manually moving the uterus away from the midline to the left side when the patient is supine. This maneuver is particularly useful when performing cardiac compressions in a pregnant patient. In the supine position, the gravid uterus, which accounts for as much as 10% of the cardiac output, hinders successful resuscitation because of its adverse effects on intrathoracic pressure and venous return. Although hemodynamics are optimized in the left lateral position, it is difficult to achieve optimal chest compressions with the patient tilted all the way into the left lateral decubitus position. Acceptable alternatives are to perform cardiac compressions with the patient supine but with concurrent manual displacement of the uterus to the other side; it is also satisfactory to place a wedge under the right hip of the patient.5,6
Oxygen Consumption and Ventricular Performance
As cardiac output progressively increases, maternal oxygen consumption also increases. However, the increase in cardiac output is seen earlier than the rise in maternal oxygen consumption. Accordingly, the arteriovenous oxygen difference actually narrows early in pregnancy. The arteriovenous oxygen difference widens at the end of gestation. By term, there is a 20% increase in maternal oxygen consumption, mostly as a result of the increase in metabolic needs of the fetus. The increase in oxygen consumption is also a result of the increased work of ventilation during pregnancy, the increase in myocardial oxygen demand, and the increase in renal oxygen consumption. Oxygen extraction also gradually increases throughout gestation. The increase in cardiac output is probably the result of a combination of factors including increased uterine blood flow, increased maternal circulating blood volume (and hence ventricular preload), and possibly estrogen- and prolactin-induced augmentation of myocardial contractility. Ventricular dynamics are improved during pregnancy as a direct result of the action of steroid hormones on the pregnant myocardium. In animal models, estrogens have been shown to increase cardiac output and decrease peripheral vascular resistance.7 Echocardiographic studies performed in healthy pregnant women have demonstrated a decrease in the pre-ejection period of left ventricular systole but an increase in the left ventricular end-diastolic dimension.8–10 It may be that a combination of improved myocardial contractility and increased ventricular diastolic area may be responsible for increases in cardiac output during normal pregnancy.11
Hemodynamic Changes during Labor and Delivery
The hemodynamic changes seen during labor and delivery are influenced by anesthetic and analgesic techniques. The increase in cardiac output is less if caudal anesthesia is used.12,13 Within the first 20 to 30 minutes after delivery of the fetus and placenta, there is an even greater increase in cardiac output, because blood is no longer diverted to the uteroplacental vascular bed. Approximately 500 mL is redirected to the maternal circulation in the so-called autotransfusion effect of pregnancy. This effect can cause cardiac output to increase by 60% to 80% after aortocaval compression is removed and blood volume is increased. Most of the physiologic changes of pregnancy resolve and revert to normal within several days after delivery. Cardiac output returns to normal within 2 weeks to 3 months after delivery as sodium and water balances normalize.
Blood Volume Changes
The changes in maternal blood volume during pregnancy are dramatic. Plasma volume increases by 30% to 50% by the end of gestation. This value is increased in the multigravida patient compared with primigravidas, but the exact mechanism responsible for this effect is unclear. The increase in blood volume can be as high as 70% with twin pregnancies. An increase of 10% to 15% in blood volume is seen as early as the seventh week of gestation. Blood volume is maximal at 30 to 34 weeks, after which the value plateaus until term.14 Ventricular filling pressures do not increase despite the large increases in plasma volume.15 This is most likely the result of concurrent decreases in systemic and pulmonary vascular resistance.
Plasma renin and aldosterone levels are elevated during pregnancy despite expansion of the maternal blood volume. Activation of the renin-angiotensin-aldosterone system may result from the concomitant decrease in peripheral vascular resistance and the increase in vascular capacitance seen as early as the first 6 weeks of pregnancy.2 Both estrogens and progesterone increase aldosterone levels, increasing sodium and water retention.16 At 12 weeks of gestation, atrial natriuretic peptide levels also increase, most likely in response to the increase in plasma volume.
Physiologic Anemia of Pregnancy
The degree of change in RBC mass during pregnancy depends in part on whether iron is supplemented. With the increase in RBC mass, there is a need for additional iron to prevent the development of iron-deficiency anemia. Maternal requirements for iron can increase to 5 to 6 mg/d. The fetus uses iron from maternal stores to prevent fetal anemia, but the presence of significant maternal iron-deficiency anemia has been shown to result in a higher incidence of fetal complications, including preterm labor and late spontaneous abortions.17
Changes in Blood Pressure and Vascular System
Arterial blood pressure decreases as early as the sixth week of pregnancy; the lowest diastolic pressures are recorded during the second trimester. By the eighth week of gestation, diastolic blood pressure decreases by approximately by 10%. Diastolic pressure reaches a nadir at 16 to 24 weeks and is typically 5 to 10 mm Hg less than normal. After the 16th gestational week, blood pressure progressively increases and is back to baseline by term. With the increase in venous return associated with uterine contractions and the additional factors of pain, anxiety, and stress during labor and delivery, an increase in blood pressure usually occurs during this time. The decrease in blood pressure during pregnancy is associated with a significant decrease in peripheral vascular resistance. The decrease in arteriolar tone is influenced by several factors, including hormonal changes that induce vasodilatation and lack of responsiveness to the pressor effect of angiotensin II.18 There is evidence for blood vessel remodeling in pregnancy, leading to increased venous compliance.19,20 During pregnancy, circulating levels of numerous endogenous procoagulant and anticoagulant proteins change, leading to a hypercoagulable state. As a consequence, the risk of venous thrombosis increases during pregnancy. The reported incidence is 0.7 cases per 1000 women, and this rate increases threefold to fourfold in the postpartum period.21
The treatment of choice for severe hypotension resulting from acute hemorrhage, sepsis, or other critical illness during pregnancy is (ideally) aggressive fluid resuscitation. However, in cases of fluid-unresponsive hypotension, vasopressors must be used to prevent detrimental consequences to both the mother and fetus as a result of inadequate uterine blood flow secondary to hypotension. Most vasopressors increase maternal blood pressure at the expense of fetal blood flow, inducing vasoconstriction of the uterine vessels. There are few human studies of these agents in pregnant women. However, animal studies animals indicate that ephedrine and dopamine increase uterine blood flow to the uteroplacental circulation while at the same time increasing maternal blood pressure.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree