166 Calcium, Magnesium, and Phosphorus
• Regulation of calcium, magnesium, and phosphorus is interrelated, and abnormalities in one are highly correlated with other electrolyte abnormalities.
• Severely depressed mental status and precipitation of arrhythmias are the most dangerous consequences of severe abnormalities in calcium, magnesium, or phosphorus.
• Proper correction of electrolyte deficiencies requires knowledge of the various oral and intravenous electrolyte preparations available.
Calcium
Approximately 99% of total body calcium is located in bone as the calcium phosphate salt hydroxyapatite. Of the remaining total body calcium, 45% is bound to albumin; 10% is complexed with circulating ions such as bicarbonate, phosphate, citrate, or sulfate1; and the remaining 45% is found in the free, ionized form. The normal range for serum calcium is 8.5 to 10.5 mg/dL, with some variability among different laboratories. The normal range of ionized (unbound) calcium is 4.5 to 5.6 mg/dL, but this is often reported in the international units (SI units) of mmol/L, with the normal range being 1.1 to 1.4 mmol/L. This ionized fraction is responsible for the physiologic actions of calcium and is not dependent on albumin levels. The total serum calcium level can be corrected for the amount of serum albumin (see the “Facts and Formulas” box), but such correction can be unreliable, so an ionized calcium level should be obtained whenever true hypercalcemia or hypocalcemia is a concern.
![]() Facts and Formulas
Facts and Formulas
| Normal serum calcium level | 8.5-10.5 mg/dL (2.1-2.6 mmol/dL) |
| Normal ionized calcium level | 4.5-5.6 mg/dL (1.1-1.4 mmol/L) |
| Normal serum magnesium level | 1.8-2.5 mg/dL (0.74-0.94 mmol/L) |
| Normal serum phosphorus level | 2.5-4.5 mg/dL (0.81-1.45 mmol/L) |
| Total serum calcium level corrected for albumin: | For every 1 g/dL in albumin, serum calcium drops 0.8 mg/dL |
| Corrected calcium (mg/dL) | Measured calcium (mg/dL) + 0.8[4.4 − albumin (g/dL)] |
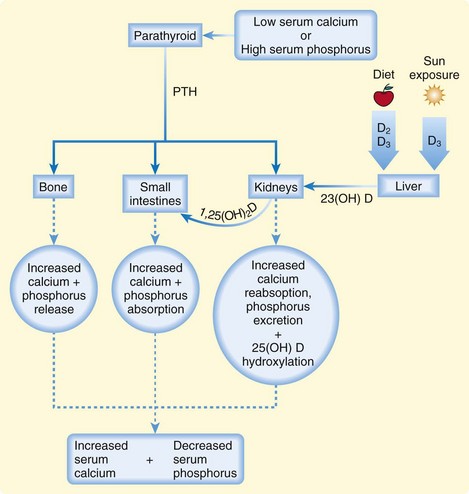
The plasma concentration of calcium is tightly maintained within the normal range by a feedback-regulated endocrine system that balances interactions among the small intestines, kidneys, bones, parathyroid glands, thyroid gland, and bloodstream. The key regulatory molecules in this system include calcium, phosphorus, parathyroid hormone (PTH), and 1,25-dihydroxyvitamin D (calcitriol)1,2 (Fig. 166.1).
Hypercalcemia
Epidemiology
The prevalence of hypercalcemia is approximately 0.5% to 3% in hospitalized adults.3 Hyperparathyroidism is the most common cause of hypercalcemia, and the incidence of primary hyperparathyroidism is approximately 21 cases per 100,000 person-years.4
The paraneoplastic syndrome hypercalcemia of malignancy is the second most common cause of hypercalcemia and occurs in approximately 10% to 30% of patients with cancer. Multiple myeloma and lung, breast, and prostate malignancies are most often associated with this disorder. It is typically seen in the end stages of disease and indicates a poor prognosis.5
Pathophysiology
Under normal conditions, excess calcium is excreted together with sodium in the proximal tubules of the kidneys. With hypercalcemia, dehydration caused by vomiting, poor oral intake, and osmotic diuresis results in reabsorption of sodium instead of excretion. This concurrent calcium reabsorption exacerbates the underlying hypercalcemia. PTH regulates the renal excretion of calcium. The excess production of PTH in primary hyperparathyroidism results in inappropriate calcium reabsorption. Causes of primary hyperparathyroidism include solitary adenomas (most common), ectopic adenomas in the mediastinum, diffuse hyperplasia of one or more parathyroid glands, and parathyroid carcinoma.6 These parathyroid abnormalities may be independent or a component of the multiple endocrine neoplasia syndromes (MEN 1 or 2a).
In the paraneoplastic syndrome hypercalcemia of malignancy, the majority of cases of hypercalcemia arise from tumor secretion of parathyroid hormone–related protein (PTHrP), a PTH homologue that acts on tissues like PTH does. Osteolytic bone metastases and ectopic tumor production of calcitriol and PTH cause the remaining cases of hypercalcemia of malignancy.5
Milk-alkali syndrome is the third most common cause of hypercalcemia severe enough to result in hospitalization.7 The clinical definition of milk-alkali syndrome is hypercalcemia, alkalosis, and renal failure in a patient ingesting excessive amounts of calcium and an alkali. Diagnosis is based on the patient history when other causes of hypercalcemia are excluded. Over-the-counter calcium carbonate supplements are commonly used for dyspepsia and prevention of osteoporosis and are currently the most frequent cause of milk-alkali syndrome. Historically, ingestion of milk and sodium bicarbonate for the treatment of peptic ulcer disease was the most common cause of milk-alkali syndrome, but this medication regimen went out of favor with the availability of H2 receptor antagonists and proton pump inhibitors. Serum PTH is low in these patients, indicative of no concurrent hyperparathyroidism.
Several medications rarely cause hypercalcemia. Thiazide diuretics, lithium, and the vitamin A derivatives all-trans-retinoic acid and cis-retinoic acid have been implicated. Some systemic illnesses also have the potential to cause hypercalcemia, including the granulomatous diseases sarcoidosis, leprosy, coccidiomycosis, histoplasmosis, and tuberculosis. The mechanism of hypercalcemia in these conditions is thought to be production of calcitriol by macrophages within granulomas.8 Additionally, rare inherited disorders such as familial hypocalciuric hypercalcemia cause hypercalcemia.9
Presenting Signs and Symptoms
Patients often become symptomatic from hypercalcemia at levels near 12 mg/dL, and nearly all patients with levels higher than 14 mg/dL will be symptomatic. Hypercalcemia affects a broad array of organ systems (Box 166.1).
A common renal manifestation of hypercalcemia is osmotic diuresis manifested as polyuria and excessive thirst. Nephrolithiasis and nephrocalcinosis are hallmarks of hypercalcemia. In patients with primary hyperparathyroidism, up to 20% have a history of symptomatic nephrolithiasis. Case series of patients with kidney stones have demonstrated a 2% to 8% incidence of primary hyperparathyroidism.10 It is thought that excessive calciuria combined with dehydration and decreased urine output leads to stone formation.
Cardiac manifestations of hypercalcemia are generally manifested as asymptomatic electrocardiographic (ECG) changes. Shortening of the QT interval (QTc <0.4 msec) is common, and ST elevations that may mimic acute myocardial infarction have been reported11,12 (Fig. 166.2). Symptomatic cardiac manifestations are rare and generally limited to bradydysrhythmias.
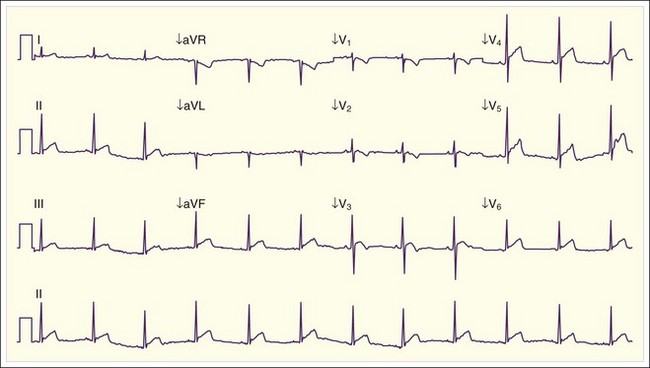
Fig. 166.2 Electrocardiographic manifestations of hypercalcemia.
Note the shortened QT interval, T-wave inversions, and ST-segment elevation.
(Courtesy Loren K. Rood, MD, Indiana University School of Medicine, Indianapolis.)
Musculoskeletal symptoms of hypercalcemia include muscle weakness, bone pain, and osteopenia.
Treatment
Initial therapy for hypercalcemia includes correction of dehydration and facilitation of renal excretion of calcium through volume reexpansion with normal saline at a rate of 200 to 500 mL/hr. Patients with severe hypercalcemia may require several liters of fluid resuscitation. For example, in a case series of patients with severe hyperparathyroid crisis requiring parathyroidectomy, a mean of 16 ± 6 L of isotonic fluid was administered over a period of several days before surgery.6
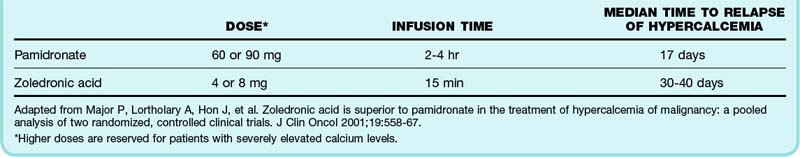
An additional therapy that has been studied most extensively in patients with hypercalcemia of malignancy is the use of bisphosphonates. These medications act on osteoclasts and limit release of calcium from bone. Their maximum calcium-lowering effects do not occur until several days after administration and can last for several weeks to months.13–15 Side effects of the bisphosphonates include hypophosphatemia, hypomagnesemia, osteonecrosis of the jaw, and postadministration acute phase reactions (fever, arthralgias, fatigue, malaise, myalgias). Table 166.1 summarizes the dosing regimens for available bisphosphonates.
Table 166.1 Bisphosphonates Available in the United States for the Treatment of Hypercalcemia of Malignancy
A treatment of hypercalcemia that is immediately effective in lowering serum calcium is calcitonin. Calcitonin inhibits urinary reabsorption of calcium and osteoclast maturation. The most commonly available form of this medication is salmon calcitonin administered at 4 to 8 U/kg subcutaneously every 8 to 12 hours. Lowering of the serum calcium level can occur as quickly as 2 hours after administration, but the effects are generally modest (lowering calcium by up to 3.8 mg/dL)16 and short-lived. Tachyphylaxis to this treatment occurs within 2 days. Side effects of salmon calcitonin include flushing, nausea, vomiting, and abdominal cramps.
Glucocorticoids inhibit conversion of 25-hydroxyvitamin D to calcitriol, which causes a decrease in intestinal absorption of calcium and an increase in renal calcium excretion. Efficacy in lowering serum calcium has been demonstrated only in the treatment of certain types of lymphoma that secrete calcitriol, vitamin D intoxication, and the granulomatous diseases.8 Additionally, administration of glucocorticoids may delay tachyphylaxis to calcitonin, so they are often used in conjunction with salmon calcitonin. A common regimen for the treatment of hypercalcemia is hydrocortisone, 200 to 300 mg/day intravenously for 3 to 5 days.
Follow-Up, Next Steps in Care, and Patient Education
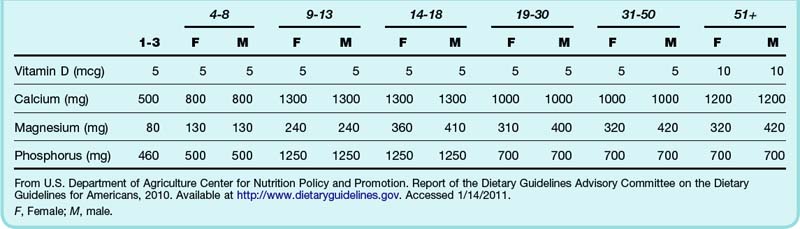
Hospital admission is indicated for patients with symptomatic hypercalcemia, especially in the setting of altered mental status, dehydration, or acute renal failure. Definitive treatment of any underlying diseases causing hypercalcemia should be undertaken, as clinically appropriate. This may include parathyroidectomy for primary hyperparathyroidism or initiation of therapy for malignancy. Patients should be educated about the proper use of dietary supplements or antacids containing calcium and vitamin D (Table 166.2).
Hypocalcemia
Epidemiology
Hypocalcemia is defined as a serum calcium level lower than 8.5 mg/dL, although symptoms of hypocalcemia typically do not occur until serum calcium is below 7.0 to 7.5 mg/dL or ionized calcium is below 2.8 mg/dL (0.7 mmol/L).17 The incidence of hypocalcemia has not been well quantified.